ORGANIC I LABORATORY KELLY SEPARATION OF LIQUIDS BY
advertisement

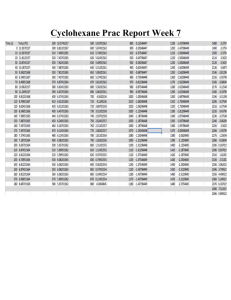
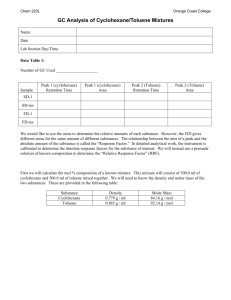
ORGANIC I LABORATORY KELLY SEPARATION OF LIQUIDS BY GAS CHROMATOGRAPHY READ: Landgrebe pp 99-106 I. Purpose: To Learn the techniques of gas-liquid chromatography by analysis of the distillation fractions collected in the last experiment II. Samples to Analyze: A. A 50:50 mixture of cyclohexane and toluene (provided at GC station) B. An unknown mixture of cyclohexane and toluene (provided at GC station) C. Fraction 1 --- the first 2 mL of distillate from previous experiment D. Fraction 2 --- the residue left in the distillation pot III. Procedure A. Check the gas chromatograph and recorder settings to see that they match those recommended on the next page under Gas Chromatograph and Recorder Settings . You will inject into the right (nonpolar) column. The stationary phase is a silicone oil which allows compounds to separate primarily by their volatility. You will determine the identity of the peaks on the basis of their retention times and the boiling points of the cyclohexane and toluene. B. Turn on the recorder chart drive. C. Flush the syringe twenty times with the sample you will inject. Never force the syringe plunger. If it sticks, ask the instructor for assistance. Inject 0.5 µL sample sizes. While injecting, insert the needle through the center of the septum, push in the plunger, and withdraw the needle - - all in one smooth action. Do not force the needle, if you encounter resistance. Withdraw it and try again. D. In each chromatogram, the largest peak should be at least half way up the chart, but not off the top of the chart. If you do not obtain this result on the first injection, reinject a second sample, adjusting the sample size or attenuation, as necessary. Increasing sample size or lowering the attenuation will increase peak size. E. When you have completed your injections, turn off the chart drive. If you are working in pairs, you must give your chromatograms to your instructor or lab assistant, to be photocopied so that each of you have the chromatogram to turn in with your reports. Separation of Liquids by Gas Chromatography V. Gas Chromatograph and Recorder Settings Page 2 GAS CHROMATOGRAPH SETTINGS Injection size: 0.75 mL Attenuation: 256 (25 in the old units) Polarity: right Right (Recorder plug cord on the on the old units) Column Temperature: Detector Temperature: D. E. F. G. low (Setting "C" on old units) Bridge Current: units) none (plug in banana plug on old Zero Control: Adjust until recorder pen is on the "1" RECORDER SETTINGS Volts: Speed (On/Off): ÷ Switch: CHT: VI. A. B. C. 80° (Setting "C" on old units) 0.001 1 1 On (when you are ready to inject) Report Cover Sheet with Experiment Title and your name Short Introduction, describe the purpose of the experiment. Chromatograms (on each of which the following must be shown) 1. The injection point (mark and label according to sample injected) 2. The identity of each peak (you may have a small air peak) 3. The type of column used (polar or nonpolar) 4. The actual temperature of the column. (Use a thermometer) 5. Your name For each calculation below, show your method of finding the solution on at least one example for each type of calculation. Calculate the areas of each peak by multiplying the width at half height times the full height of each peak. Show, by each peak on the chromatograms, the numbers you get. The detector will not be equally sensitive to both compounds. You can see that by looking at the relative areas for toluene and cyclohexane in the 50:50 mixture. Determine the relative molar response (RMR) of the toluene peak relative to the cyclohexane peak using the chromatogram of the 50:50 mixture. (The RMR for toluene relative to cyclohexane is just the ratio of the toluene peak area to the cyclohexane peak area.) Then all cyclohexane peaks must be corrected by multiplying their areas by the toluene RMR value. Calculate the percent cyclohexane in each sample using the formula below. area corrected CH peak % cyclohexane (CH) = [area corrected CH peak] + [area of Tol peak] x 100 Separation of Liquids by Gas Chromatography Page 3 H. Summarize your calculations in a table which shows the following information for each chromatographic analysis. Sample Identity Area for Cyclohexane Area for Corrected Cyclohexane Area for Toluene % Cyclohexane I. Calculate the number of theoretical plates per foot of GC column using the formula on page 101 of your lab manual. Use the peak for cyclohexane in the 1st fraction from your distillation experiment. The GC column is 5 feet long. VII. Study Questions for the exam. 1. As a mixture of an alcohol and a hydrocarbon passes down a polar gas chromatography column, one of the compounds will move out ahead of the other. Discuss the physical changes which are occuring that cause the one compound to move through the column faster. This will probably take a half a page to cover adequately. 2. Why does a nonpolar substrate on a gas chromatography column always separate compounds on the basis of boiling point only? 3. Explain why it was necessary to correct the peak area for the cyclohexane before it was used in your calculations for the % cyclohexane. 4. It would have been possible to have corrected the peak area for the toluene rather than the cyclohexane and still obtain the same answer for the % cyclohexane. Do the calculation this way for your unknown. Show your method of calculation for each calculation used. 5. How does the gas chromatograph detect compounds when they exit the column. 6. To what type of compounds is gas chromatography limited? 7. Be able to show a schematic of a gas chromatograph and explain the function of each part. (see Figure 6.1, page 82, in lab text) 8. Understand the technique of mixed injections and how it can be used to provide evidence for the identity of a peak in a chromatogram