Study Problems Ch 12. Infrared Absorption and Chemical Structure
advertisement
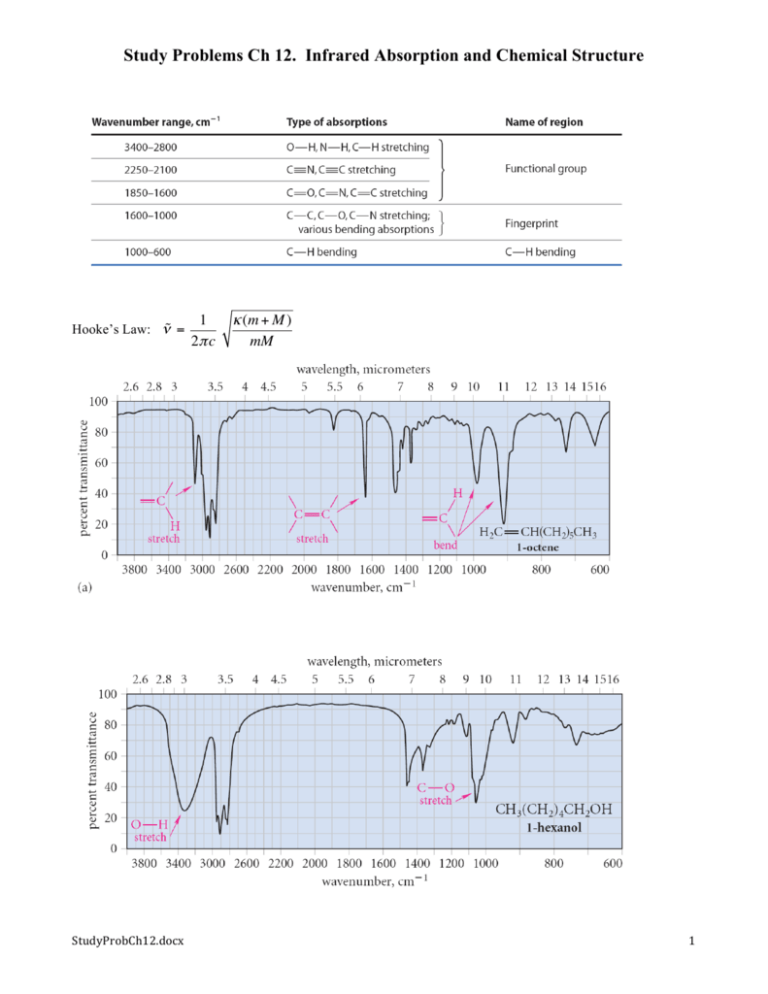
Study Problems Ch 12. Infrared Absorption and Chemical Structure Hooke’s Law: ν = StudyProbCh12.docx 1 κ (m + M ) 2π c mM 1 StudyProbCh12.docx 2 Chapter 12 Study Problems. Introduction to Spectroscopy: IR 12.7 The following bonds all have IR stretching absorptions in the 4000–2900 cm-1 region of the spectrum. Rank the following bonds in order of decreasing stretching frequencies, greatest first, and explain your reasoning. (Hint: Consult Table 5.3 on p. 213.) C—H, O—H, N—H, F—H 12.8 The =C—H stretching absorption of 2-methyl-1-pentene is observed at 3090 cm-1. If the hydrogen were replaced by deuterium, at what wavenumber would the =C—D stretching absorption be observed? Explain. (Assume that the force constants for the C—H and C—D bonds are identical.) 12.9 Which of the following vibrations should be infrared-active and which should be infrared-inactive (or nearly so)? (a) CH3 CH2 CH2 CH2 C CH (b) (CH3)2C=O C C stretch C=O stretch (c) Cyclohexane ring “breathing” (simultaneous stretch of all C-C bonds) D (d) CH3 CH2 C C CH2 CH3 C C stretch O (e) H3C symmetrical N-O stretch + N O - (f) (CH3) 3C—Cl C—Cl stretch (g) trans-3-hexene C=C stretch StudyProbCh12.docx 3 Study Problem 12.3 Each of three alkenes, A, B, and C, has the molecular formula C5H10, and each undergoes catalytic hydrogenation to yield pentane. Alkene A has IR absorptions at 1642, 990, and 911 cm-1; alkene B has an IR absorption at 964 cm-1, and no absorption in the 1600–1700 cm-1 region; and alkene C has absorptions at 1658 and 695 cm-1. Identify the three alkenes. 12.10 Five isomeric alkenes A –E each undergo catalytic hydrogenation to give 2-methylpentane. The IR spectra of these five alkenes have the following key absorptions (in cm-1): Compound A: 912 (s), 994 (s), 1643 (s), 3077 (m) Compound B: 833 (s), 1667 (w), 3050 (weak shoulder on C—H absorption) Compound C: 714 (s), 1665 (w), 3010 (m) Compound D: 885 (s), 1650 (m), 3086 (m) Compound E: 967 (s), no absorption 1600–1700, 3040 (m) Propose a structure for each alkene. 12.11 One of the spectra in Fig. 12.11 (p. 556) is that of trans-2-heptene and the other is that of 2methyl-1-hexene. Which is which? Explain. StudyProbCh12.docx 4 12.12 Match the IR spectrum in Fig. 12.13 to one of the following three compounds: 2-methyl-1octene, butyl methyl ether, or 1-pentanol. 12.13 Explain why the IR spectra of some ethers have two C—O stretching absorptions. (Hint: See Fig. 12.8, p. 549.) 12.14 Explain why the frequency of the O—H stretching absorption of an alcohol in solution changes as the alcohol solution is diluted. 12.22 List the factors that determine the wavenumber of an infrared absorption. 12.23 List two factors that determine the intensity of an infrared absorption. 12.24 Indicate how you would carry out each of the following chemical transformations. What are some of the changes in the infrared spectrum that could be used to indicate whether the reaction has proceeded as indicated? (Your answer can include disappearance as well as appearance of IR absorptions.) (a) 1-methylcyclohexene → methylcyclohexane 12.25 Which of the molecules in each of the following pairs should have identical IR spectra, and which should have different IR spectra (if only slightly different)? Explain your reasoning carefully. (a) 3-pentanol and (± )-2-pentanol (b) (R)-2-pentanol and (S)-2-pentanol (c) and StudyProbCh12.docx 5 12.26 Match each of the IR spectra in Fig. P12.26 to one of the following compounds. (Notice that there is no spectrum for two of the compounds.) (a) 1,5-hexadiene (b) 1-methylcyclopentene (c) 1-hexen-3-ol (d) dipropyl ether (e) trans-4-octene (f ) cyclohexane (g) 3-hexanol StudyProbCh12.docx 6 12.27 A former theological student, Heavn Hardley, has turned to chemistry and, during his eighth year of graduate study, has carried out the following reaction: H2, catalyst O O A B Unfortunately, Hardley thinks he may have mislabeled his samples of A and B, but has wisely decided to take an IR spectrum of each sample. The spectra are reproduced in Fig. P12.27 on p. 574. Which sample goes with which spectrum? How do you know? 12.28 (a) Given the stretching frequencies for the C—H bonds shown in color, arrange the corresponding bonds in order of increasing strength. Explain your reasoning. H H R CH CH 3080 cm-1 R CH2 RC 2850 cm-1 C H 3300 cm-1 (b) If the bond dissociation energy of the ≡C-H bond is 548 kJ mol-1 (131 kcal mol-1), use the stretching frequencies in part (a) to estimate the bond dissociation energy of the C—H bond in RCH2—H. StudyProbCh12.docx 7 12.29 Arrange the following bonds in order of increasing stretching frequencies, and explain your reasoning. C=C C≡C C=O C—C 12.30 (a) The water molecule has three distinguishable molecular vibrations. Construct a diagram like Fig. 12.8 on p. 549 for the three vibrational modes of water. (Hint: Each vibration at its extremes must change the molecule so that it can be distinguished from the molecule before the vibration occurs.) (b) Classify each vibration as a stretching or bending vibration. (c) The IR spectrum of water vapor has three absorptions: 1595, 3652, and 3756 cm-1. Which are stretching vibrations and which are bending vibrations? Explain. 12.31 Explain why a nitro compound has two N—O stretching vibrations. (These typically occur at about 1370 and 1550 cm-1.) 12.32 (a) Explain why the S—H stretching absorption in the IR spectrum of a thiol is less intense and occurs at lower frequency (2550 cm-1) than the O—H stretching absorption of an alcohol. (b) Two unlabeled bottles, A and B, contain liquids. Laboratory notes suggest that one compound is (HSCH2CH2) 2O and the other is (HOCH2CH2) 2S. The IR spectra of the two compounds are given in Fig. P12.32. Identify A and B and explain your choice. StudyProbCh12.docx 8 12.33 (a) You have found in the laboratory two liquids, C and D, in unlabeled bottles. You suspect that one is deuterated chloroform (CDCl3) and the other is ordinary chloroform (CHCl3). Unfortunately, the mass spectrometer is not operating because the same person who failed to label the bottles has been recently using the mass spectrometer! From the IR spectra of the two compounds, shown in Fig. P12.33 on p. 576, indicate which compound is which. Explain. StudyProbCh12.docx 9