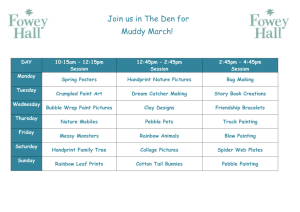
Posters - NCDEU - ASCP Annual Meeting
advertisement

NEW APPROACHES TO MENTAL ILLNESS IN THE ERA OF THE NATIONAL BRAIN INITIATIVE ASCP ANNUAL MEETING June 16 - 19, 2014 HOLLYWOOD, FL www.ASCPMeeting.org Dear Colleagues: On behalf of the American Society of Clinical Psychopharmacology (ASCP), we are very pleased to welcome you to this year’s “ASCP Annual Meeting.” In 2010, several decisions were made that allowed ASCP to reinvent NCDEU as “the New NCDEU.” Over the last three years “the New NCDEU” has been quite successful. Moving forward in 2014, the ASCP Board and the ASCP/NCDEU Steering Committee have decided to rename the meeting the “ASCP Annual Meeting.” The ASCP is proud to sponsor the meeting, now in its 54th year. Psychopharmacology is an exciting field undergoing dramatic changes, with the identification of new molecular targets and the development of novel compounds. Our Society keeps its members abreast of such innovations. Our annual meeting serves as the vehicle for the dissemination of cutting edge psychopharmacology research. We will continue to develop ways for members to feel more connected with our Society and to include more clinically focused symposia. Another major goal for ASCP is to increase its visibility to those interested in the practice of psychopharmacology, thereby helping our membership grow. With over 1,000 annual attendees, the meeting has also become a key opportunity for networking, planning and to develop the next generation of clinical researchers. A very special thank you to the members of the ASCP/NCDEU Steering and ASCP Program Committees for their critical role in the success of the meeting. We are all looking forward to an exciting and highly informative ASCP Annual Meeting. Maurizio Fava, M.D. President American Society of Clinical Psychopharmacology Welcome to the ASCP Annual Meeting On behalf of the ASCP Annual Meeting Steering and Program Committees, we are delighted to welcome you to the ASCP Annual Meeting. Though the name of the meeting has changed, the ASCP is committed to continue to build on the past success of NCDEU with program innovation while preserving the rich history of this meeting. Below are some of the highlights of the 2014 meeting. The annual meeting brings together academic investigators, industry scientists, U.S. and international regulators, National Institutes of Health (NIH) and other professionals who work in drug development and clinical trials. • 2014 Program Highlights oMonday, June 16 §Conference Opening §Pharma Pipeline: 10 presentations of Phase 1 and Phase 2 developments. oTuesday, June 17 §15th Annual Fun Run/Walk §Regulatory Plenary: FDA Science Initiatives: A Brief Update §ASCP Lifetime Awardee Talk §Poster Session I §ASCP Reception oWednesday, June 18 §Keynote Plenary Session: New Approaches to Mental Illness in the Era of the National Brain Initiative §NIH Institute Directors Plenary §Poster Session II §Updates Session – The Latest on Treatment of Mood, OCD-spectrum, and Binge Eating Disorders §Workshops oThursday, June 19 §Regulatory Wrap-Up Plenary with the FDA oThroughout the meeting §NIMH, NIDA, NCCAM, and NIAAA panels oThe New Investigator Program §A closed workshop for 20 New Investigators and informal breakfast sessions oWorkshops: 2 hour intensive interactive sessions focused on problems and solutions §Tuesday and Wednesday Afternoons o*Clinical Track* – sessions focused on topics of immediate clinical relevance • Organization oThe meeting is sponsored by the American Society for Clinical Psychopharmacology (ASCP). §The Steering Committee organizes the meeting. §The Program Committee evaluates submitted proposals and develops program innovations. oNIH collaborations: §NIMH - National Institute of Mental Health §NIDA - National Institute of Drug Abuse §NIAAA National Institute on Alcohol Abuse and Alcoholism §NCCAM - National Center for Complementary and Alternative Medicine §CSR – Center for Scientific Review §NCATS - National Center for Advancing Translational Sciences oRegulatory agency collaborations: §Food and Drug Administration (FDA) oParthenon Management Group organizes the ASCP Annual Meeting. • And remember oThe Opening Reception is Tuesday, June 17th from 7:00 pm – 8:00 pm. oThe Fun Run/Walk is Tuesday, June 17th at 6:45 am. The ASCP Annual Meeting is an opportunity for education and networking. We welcome your suggestions to make the event even better. Seek us out during the meeting or provide your views by completing the evaluation form. Best Regards, Husseini Manji, M.D., FRCPC Steering Committee Co-Chair Michael E. Thase, M.D. Steering Committee Co-Chair Carlos Zarate, M.D. Program Committee Co-Chair Holly A. Swartz, M.D. Program Committee Co-Chair Hotel Maps 2nd Floor Convention Center GRAND BALLROOM WEST ATLANTIC BALLROOM 3 SERVICE CORRIDOR GRAND BALLROOM EAST REGENCY BALLROOM 3 ATLANTIC BALLROOM 2 REGENCY BALLROOM 2 ATLANTIC BALLROOM 1 M REGISTRATION CONVENTION CENTER THE WESTIN DIPLOMAT FREIGHT ELEVATOR REGENCY BALLROOM 1 W 5 SERVICE CORRIDOR SERVICE ELEVATORS 4 DIPLOMAT BALLROOM 3 1 2 S ESCALATOR ELEVATORS W E TERRACE N WALKWAY TO RESORT Resort, Second Floor NORTH TOWER TER ACE 203 202 204 SOUTH TOWER RAC TERR 220 201 E CONVENTION CENTER THE WESTIN DIPLOMAT 218 219 217 ESCALATORS 216 205 SERVICE ELEVATORS SERVICE ELEVATORS ELEVATORS M W 206 SKYWALK WALKWAY TO CONVENTION CENTER ELEVATORS W 209 M 215 208 E 207 EXECUTIVE OFFICES 212 BUSINESS CENTER TERRACE 213 214 S N W 2 Hotel Maps Resort, Third Floor NORTH TOWER 302 303 RAC E 320 301 322 305 SOUTH TOWER TER CE A TERR THE WESTIN DIPLOMAT CONVENTION CENTER 318 319 317 321 ESCALATOR 316 ESCALATOR SERVICE ELEVATORS SERVICE ELEVATORS ELEVATORS M W 306 SKYWALK ELEVATORS 309 310 308 311 W M 315 E 312 307 313 314 S N EXECUTIVE OFFICES TERRACE W Future ASCP Meetings • State of the Art – Fall Psychopharmacology Update Meeting October 11-12, 2014 New York, New York • 2015 ASCP Annual Meeting June 22-25, 2015 Miami Beach, Florida 3 Table of Contents Award Winners and Featured Speakers............................................5 Acknowledgements.......................................................................15 Meeting Announcements...............................................................21 Sunday, June 15, 2014...................................................................25 Monday, June 16, 2014.................................................................27 Tuesday, June 17, 2014..................................................................41 Wednesday, June 18, 2014............................................................53 Thursday, June 19, 2014................................................................63 Poster Session 1.............................................................................69 Poster Session 2.............................................................................81 Author Index.................................................................................93 DISCLOSURES FOR ALL ASCP PRESENTERS CAN BE VIEWED AT WWW.ASCPMEETING.ORG 4 Recipient of the Donald Klein Lifetime Achievement Award A. John Rush, M.D. Duke-National University of Singapore Dr. Rush majored in Biochemistry at Princeton and completed his M.D. at Columbia College of Physicians and Surgeons. Following his internship in general medicine (Passavant Hospital, Northwestern University), he served in the U.S. Army before joining the Special Action Office for Drug Abuse Prevention in Washington D.C. After his psychiatric residency at the University of Pennsylvania, he became Assistant Professor at the University of Oklahoma and subsequently joined the Departments of Psychiatry and later the Department of Clinical Sciences at the University of Texas Southwestern Medical Center at Dallas. In 2008, he became Vice Dean of Clinical Sciences and Professor at Duke-NUS (Singapore). At Duke-NUS he founded and developed several programs designed to advance patient oriented research careers of medical, surgical and mental health, nursing and allied health practitioners including the Academic Medicine Research Institute, the Center for Quantitative Medicine, and the Medical Student III program in clinical research. His research has focused on the development and testing of innovative treatments for mood disorders including medications, medication combinations, somatic treatments, psychotherapy, and disease management protocols (treatment algorithms). He has authored more than 600 papers and chapters and 10 books. He was Principal Investigator on the NIMH sponsored STAR*D (Sequenced Treatment Alternatives to Relieve Depression) trial and directed the NIMH Depression Trials Network (DTN). Past awards include the Mood Disorders Research Award (American College of Psychiatrists), the Paul Hoch Award (American Psychopathological Association), the Edward J. Sachar Award (Columbia College of P & S), the Nola Maddox Falcone Prize (NARSAD), the American Psychiatric Association Award for Research in Psychiatry, and the Gold Medal Award (Society of Biological Psychiatry). He has served as President of the Society of Biological Psychiatry and Society for Psychotherapy Research. He presently provides consultation to academic centers, government and industrial organizations and entities. 5 Awards Winners & Featured Speakers Award Winners Award Winners Recipient of the Paul Wender Best Paper in the Journal of Clinical Psychiatry Award Sophie Grigoriadias, M.D. Sunnybrook Health Sciences Centre Nominated for: Antidepressant Exposure During Pregnancy and Congenital Malformations: Is There an Association? A Systematic Review and Meta-Analysis of the Best Evidence Sophie Grigoriadis, M.D., Ph.D., FRCPC; Emily H. VonderPorten, MPH; Lana Mamisashvili, MSW; Michael Roerecke, Ph.D.; Jürgen Rehm, Ph.D.; Cindy-Lee Dennis, Ph.D.; Gideon Koren, M.D., FRCPC, FACMT; Meir Steiner, M.D., Ph.D., FRCPC; Patricia Mousmanis, M.D., CCFP, FCFP; Amy Cheung, M.D., MSc, FRCPC; and Lori E. Ross, Ph.D. Dr. Grigoriadis is the Head of the Women’s Mood and Anxiety Clinic: Reproductive Transitions at Sunnybrook health Sciences Centre and scientist at the Sunnybrook Research Institute. She was the Academic Leader of the Reproductive Life Stages Program at Women’s College Hospital, Fellowship Director in the department of psychiatry at the University Health Network (UHN) and a Staff Psychiatrist at both sites including the Mood Disorders Psychopharmacology Unit at UHN until September 2011. She is an Associate Professor in the Mood and Anxiety Disorders and Women’s Mental Health Programs, Department of Psychiatry, Faculty of Medicine, at the University of Toronto. Dr. Grigoriadis completed her medical degree at McMaster University and her psychiatry residency at the University of Toronto. Prior to Medical school, she completed a MA and Ph.D. in Clinical Psychology at Queen’s University, and internship in Clinical Psychology at the Toronto Hospital. Dr. Grigoriadis’ clinical interests include women with depression especially during pregnancy, the postpartum period and during perimenopause. Her treatment strategies include both the use of pharmacotherapy as well as short-term psychotherapies. Current research focuses on the presentation of mood disorders in women across the lifespan, hormonal influences in the presentation and treatment of depression during the postpartum and perimenopause, and differences in the presentation and response to antidepressant medications among women of various ages. Dr. Grigoriadis is also actively involved in developing novel treatment strategies for depression. She is currently leading several studies including developing a tool to assist clinicians in making treatment decisions with their depressed perinatal patients and is developing treatment guidelines for the treatment of depression during the perimenopause. She is an author in the Canadian Guidelines for the Treatment of Depression. Her educational activities include teaching psychiatry residents, training family practice as well as psychiatry residents in Interpersonal Psychotherapy and teaching in continuing medical education programs. 6 Featured Speakers Regulatory Plenary - FDA Science Initiatives: A Brief Update Tuesday, June 17, 2014 from 8:30 am – 10:00 am Ni Khin, M.D. Food and Drug Administration Dr. Ni Khin is Acting Division Director in Division of Good Clinical Practice Compliance, Office of Scientific Investigations, Center for Drug Evaluation and Research, FDA. She has served as Medical Team Leader in the Division of Psychiatry Products, Office of New Drugs for past 8 years. In this position, she oversaw the review of clinical protocols submitted under investigational new drug applications (INDs) for all phases of drug development. She also managed, as cross-disciplinary team leader, regulatory and clinical reviews of New Drug Applications (NDAs) for all psychiatric indications. She joined the Agency in 2001 as a Clinical Reviewer in the Division of Neuropharmacologic Drug Products. She also worked as Medical Officer and Branch Chief of Good Clinical Practice Branch 1, Division of Scientific Investigations, where she provided scientific oversight for CDERassigned bioresearch monitoring (BIMO) activities. She conducted on-site data-audit inspections of clinical trial sites both in the US and abroad. Prior to coming to FDA, Dr. Khin was a Senior Staff Fellow in the Geriatric Psychiatry Branch at the National Institute of Mental Health. Dr. Khin received her medical degree from the Institute of Medicine I, Rangoon, Burma (Myanmar). She completed residency training in Psychiatry at the State University of New York, Buffalo. She is board-certified in Psychiatry by the American Board of Psychiatry and Neurology. She also received a Master of Science Degree from Arizona State University. Her regulatory research interest includes trial design and methodology to improve detection of efficacy signals as well as regulatory and scientific issues regarding use of foreign data from global trials in support of NDA’s. Celia Winchell, M.D. Food and Drug Administration Celia Winchell is the Medical Team Leader for Addiction Products in the FDA’s Division of Anesthesia, Analgesia, and Addiction Products in the Center for Drug Evaluation and Research. Since 1995, Dr. Winchell has been providing regulatory oversight for drug development in all aspects of addiction treatment, and for academic research involving drugs of abuse. Dr. Winchell holds a bachelor’s degree in psychology from Harvard University, and a medical degree from the University of Virginia. She completed residency training in psychiatry at Johns Hopkins Hospital. 7 Featured Speakers Silvana Borges, M.D. Food and Drug Administration Dr. Borges received her medical degree from the State University School of Medicine in Uruguay. She completed her medical residency and is board certified in Child & Adolescent Psychiatry. She joined the Department of Pharmacology and Therapeutics in the State University School of Medicine as an Assistant Professor and then became an Assistant Professor and Founding Member of the “National Center for Drug Safety” in Uruguay. She was a Scholar at the Catalan Institute of Pharmacology (Barcelona, Spain) focusing her training in drug safety and pharmacoepidemiology. She received the Merck Foundation International Fellowship in Clinical Pharmacology Award and completed a fellowship in clinical pharmacology and pharmacogenetics at Indiana University, being mentored by Dr. David Flockhart. She was the recipient of the American Society for Clinical Pharmacology and Therapeutics Presidential Trainee Award for her work on the role of CYP2D6 genetic polymorphism on tamoxifen metabolism and its interaction with antidepressants. She joined the FDA in 2007 and is currently an Acting Clinical Team Leader with the Division of Psychiatry Products, Office of New Drugs, Center for Drug Evaluation and Research. Plenary: New Approaches to Mental Illness in the Era of the National Brain Initiative Wednesday, June 18, 2014 from 8:15 am - 9:45 am Husseini Manji, M.D., FRCPC Johnson & Johnson Pharmaceutical Research & Development Husseini K. Manji, M.D., FRCPC, is Global Therapeutic Head for Neuroscience at Janssen Research & Development, LLC, a division of Johnson & Johnson. Previously, he was Chief, Laboratory of Molecular Pathophysiology & Experimental Therapeutics, NIH, and Director of the NIH Mood and Anxiety Disorders Program. Dr. Manji received his B.S. and M.D. from the University of British Columbia. He completed fellowship training at the NIMH and completed additional training in cellular and molecular biology. His research has focused on investigation of disease-and treatment-induced changes in gene and protein networks that regulate synaptic and neural plasticity. His work has led to investigation of novel therapeutics for patients with refractory neuropsychiatric illnesses. Dr. Manji has also been involved in medical and postgraduate neuroscience education and has published extensively on the molecular and cellular neurobiology of neuropsychiatric disorders and the development of novel therapeutics. Dr. Manji has received numerous distinguished scientific and academic awards, including the NIMH Director’s Career Award for Significant Scientific Achievement, and was inducted in to the U.S. Institute of Medicine of the National Academies in 2008. He has served as Chair of the American College of Neuropsychopharmacology, is a Counselor to the Society of Biological Psychiatry and serves on a variety of editorial boards of scholarly journals. He holds voluntary leadership positions in many organizations devoted to advancement of neuroscience and advocacy for people with neuropsychiatric illnesses. He has been a member of the Howard Hughes Medical Institute and NIH Research Scholars Program Advisory Committee. 8 Featured Speakers Thomas Insel, M.D. National Institute of Mental Health Thomas R. Insel, M.D., is Director of the National Institute of Mental Health (NIMH), the component of the National Institutes of Health charged with generating knowledge to understand, treat and prevent mental disorders. His tenure at NIMH has been distinguished by groundbreaking findings on the genetics and neurobiology of mental disorders ads well as efforts to transform the diagnosis and treatment of serious mental illnesses. In addition to his leadership of the NIMH, Dr. Insel has served as Chair of the Interagency Autism Coordinating Committee (Since 2002), co-chair of the NIH Blueprint for Neuroscience Research (since 2004), and Acting Director of the National Center for Advancing Translational Science (2011-2012). Currently, Dr. Insel is on the leaders for the NIH Brain Research through Advancing Innovative Neurotechnologies (BRAIN) effort, a Presidential initiative focused on developing new tools for understanding the brain. Prior to his appointment as NIHM Director in the Fall 2002, Dr. Insel was Professor of Psychiatry at Emory University. There, he was founding director of the Center for Behavioral Neuroscience and director of an NIH-funded Center for Autism Research. From 1994 to 1999, he was Director of the Yerkes Regional Primate Research Center in Atlanta. While at Emory, Dr. Insel continued the line of research he had initiated at NIMH, studying the neurobiology of complex social behaviors. He has published over 280 scientific articles and four books, including the Neurobiology of Parental Care (with Michael Numan) in 2003. Dr. Insel is a member of the Institute of Medicine, a fellow of the American College of Neuropsychopharmacology, and is a recipient of several awards, including the Outstanding Service Award from the U.S. Public Health Service and the 2010 La Foundation IPSEN Neuronal Plasticity Prize. Dr. Insel graduated from the combined B.A.-M.D. program at Boston University in 1974. He did his internship at Berkshire Medical Center, Pittsfield, Massachusetts, and his residency at the Langley Porter Neuropsychiatric Institute at the University of California, San Francisco. 9 Featured Speakers Patrick Kennedy Former US Representative and Mental Health Activist Patrick J. Kennedy became the youngest member of the Kennedy family to hold elected office when, in 1988, he won election to the Rhode Island House of Representatives at age 21. Since then, Kennedy went on to serve 16 years in the United States House of Representatives, representing Rhode Island’s first congressional district from 1994 to 2011. While in office, he distinguished himself as a leader on issues of healthcare, sciences, veterans, technology, civil rights, and mental health. As a founding member of the 21st Century Healthcare Caucus; the Addiction, Treatment and Recovery Caucus; and the Down Syndrome Caucus, Kennedy has been a tireless advocate for access to health and advancements in medical research. Throughout his career, Kennedy has been a vocal advocate for healthcare reform. He was the author and chief House sponsor of the Wellstone-Domenici Mental Health Parity and Addiction Equity Act of 2008, an act that expanded access to mental health services to over 100 million Americans. He has also authored and cosponsored dozens of bills to increase the understanding and treatment of neurological and psychiatric disorders, including the National Neurotechnology Initiative Act, the Genomics and Personalized Medicine Act, the Combat PTSD Act, and the Alzheimer’s Treatment and Caregiver Support Act. Since leaving office in 2011, Patrick J. Kennedy has been devoting his efforts to promoting research in neuroscience. He co-founded One Mind for Research, a nonprofit organization, the mission of which is to be the leader in brain research in order to eliminate stigma, transform policy, and allocate resources that will help both our understanding and treatment of brain diseases. In addition to One Mind for Research, Kennedy is also an active board member of Best Buddies, Research America, and the Edward M. Kennedy Institute for the United States Senate. The champion of mental health in Congress who believes that “the brain is the last medical frontier.” Patrick J. Kennedy is a sought-after speaker on mental health, healthcare, and many other related issues. He was invited by House Minority Leader Nancy Pelosi to address the Democratic Caucus on mental health and will launch the Kennedy Forum on Community Mental Health. In 2013, he spoke at the National Institute of Mental Health Alliance for Research Progress, the US/Canada Forum on Mental Health and Productivity, and the Mental Health America Conference. 10 Featured Speakers Institute Directors Plenary Wednesday, June 18, 2014 from 10:00 am – 12:00 pm Thomas Insel, M.D. National Institute of Mental Health See previous bio Phillip Skolnick, Ph.D., D.Sc. (hon.) National Institute on Drug Abuse Phil Skolnick is the Director, Division of Pharmacotherapies and Medical Consequences of Drug Abuse at the National Institute on Drug Abuse, NIH. Dr. Skolnick served as Chief Scientific Officer (2001-2009) and President (2007-2009) of DOV Pharmaceutical, Inc. He was also Research Professor of Psychiatry (2001-2009) and a member of the Center of Excellence on Drug Addiction at New York University-Langone Medical Center. Dr. Skolnick was a Lilly Research Fellow (Neuroscience) at Lilly Research Laboratories (1997-2000). Prior to this, he was a Senior Investigator and Chief, Laboratory of Neuroscience, at the NIH intramural research program (1986-1997). Dr. Skolnick has also served as a Research Professor of Psychiatry at the Uniformed Services University of the Health Sciences, Adjunct Professor of Anesthesiology at Johns Hopkins University, and Adjunct Professor of Pharmacology and Toxicology at Indiana University School of Medicine. He received a Ph.D. from the Department of Pharmacology, George Washington University School of Medicine (1972), and served as a Staff Fellow and Senior Staff Fellow at the NIH under Dr. John W. Daly (1972-1977). His awards and honors include the Experimental Therapeutics Prize from the American Society for Pharmacology and Experimental Therapeutics, an Anna Monika Prize, and the A.E. Bennett Award in Biological Psychiatry. He has twice been awarded the Doctor of Science, honoris causa. Dr. Skolnick has co-authored more than 500 articles and currently serves on the editorial advisory board of seven journals. He is currently an editor of Current Protocols in Neuroscience, and has also edited six books. The Institute of Scientific Information (ISI) has acknowledged his contributions by naming him to the elite group of “Highly Cited” authors. 11 Featured Speakers Kenneth Warren, Ph.D., D.Sc. National Institute on Alcohol Abuse and Alcoholism Kenneth R. Warren, Ph.D., a nationally-recognized expert on alcohol and pregnancy, and a long-time senior administrator at the National Institute on Alcohol Abuse and Alcoholism (NIAAA) became Acting Director of NIAAA on November 1, 2008, following the retirement of Ting-Kai Li, M.D. on October 31, 2008. Dr. Li had served as NIAAA Director from September 2002 through October 2008. Dr. Warren was named as the NIAAA Deputy Director in February 2008. He held numerous positions in NIAAA since joining the Institute in 1976. A graduate of the City College of New York, Dr. Warren earned his doctorate degree in Biochemistry from Michigan State University in 1970. He subsequently undertook postdoctoral positions at the University of California, Los Angeles and at University of Michigan Mental Health Research Institute before joining the Federal government in a research position at the Walter Reed Army Institute of Research in 1974. Dr. Warren has maintained an active interest in all areas of alcohol and health and in past years often served as the editor of the triennial Reports to Congress on Alcohol and Health. He has been particularly active in research on the effects of alcohol use during pregnancy, including fetal alcohol syndrome (FAS) and fetal alcohol spectrum disorders (FASD). Dr. Warren initiated NIAAA’s research program on FAS over 30 years ago. He currently chairs the government-wide Interagency Coordinating Committee on FAS. Dr. Warren has received numerous honors, including a superior service award from the Public Health Service in 1982 for his work in development of the first Surgeon General’s Advisory on FAS. In 2007, the National Organization on Fetal Alcohol Syndrome (NOFAS) honored Dr. Warren by placing his name into their Tom and Linda Daschle FASD Hall of Fame, followed by the receipt of the NOFAS Excellence Award in 2008. Christopher P. Austin, M.D. National Center for Advancing Transitional Sciences In September 2012, NIH Director Francis S. Collins announced the appointment of Christopher P. Austin, M.D., as director of the National Center for Advancing Translational Sciences (NCATS). Austin succeeded former acting director of NCATS and current director of the National Institute of Mental Health Thomas R. Insel, M.D. Austin, who served as director of the NCATS Division of Pre-clinical Innovation since the creation of the Center in December 2011, is leading NCATS in its mission to catalyze the generation of innovative methods and technologies that will enhance the development, testing and implementation of diagnostics and therapeutics across a wide range of human diseases and conditions. Currently, many costly, time-consuming bottlenecks exist in the translational pipeline. Austin is applying his experiences in nearly every stage of the research pipeline to build on the Center’s momentum in finding innovative ways to reduce, remove or bypass these bottlenecks and speed the delivery of new drugs, diagnostics and medical devices to patients. 12 Featured Speakers After working at Merck on genome-based discovery of novel targets and drugs, Austin began his NIH career in 2002 as the Senior Advisor to the Director for translational research at the National Human Genome Research Institute, where he initiated the Knockout Mouse Project and the Molecular Libraries Roadmap Initiative. Other NIH roles have included serving as Director of the Therapeutics for Rare and Neglected Diseases program as well as the NIH Chemical Genomics Center and as Scientific Director of the NIH Center for Translational Therapeutics. Austin earned a medical degree from Harvard Medical School and an undergraduate degree in biology from Princeton University. He completed clinical training in internal medicine and neurology at Massachusetts General Hospital as well as a fellowship in genetics at Harvard. Richard Nakaruma, M.D. Center for Scientific Review Dr. Richard K. Nakamura is the Director of the Center for Scientific Review. In that capacity, he leads the review of grant applications of the National Institutes of Health. Dr. Nakamura received his Bachelor of Arts in Psychology from Earlham College and his Ph.D. in Psychology from State University of New York (Stony Brook, NY). He was with the National Institute of Mental Health from 1976 to 2011. In 2001, he received the NIH-Asian/Pacific American Organization (APAO) Outstanding Achievement Award for Administrative Work. In 2002, Dr. Nakamura was elected by the American Association for the Advancement of Science (AAAS) to the status of AAAS Fellow. Also in 2002, Dr. Nakamura was awarded the Presidential Rank Award for outstanding leadership. In 2004 and 2005, respectively, he received leadership awards from the Federation of Behavioral Psychological and Cognitive Sciences, and from the International Society for Behavioral Neuroscience. In 2009, he was awarded the NIH Director’s Award for Outstanding Administration. Josephine Briggs, M.D. National Center for Complementary and Alternative Medicine An accomplished researcher and physician, Dr. Briggs received her A.B. in biology from Harvard-Radcliffe College and her M.D. from Harvard Medical School. Previous to her appointment at NCCAM, she was director of the Division of Kidney, Urologic, and Hematologic Diseases at the NIH National Institute of Diabetes and Digestive and Kidney Diseases. In 2006, Dr. Briggs became Senior Scientific Officer at the Howard Hughes Medical Institute. In January 2008, she returned to NIH as the NCCAM Director. 13 Featured Speakers Regulatory Wrap-Up Thursday, June 19, 2014 from 10:15 am – 11:45 am Ni Khin, M.D. Food and Drug Administration See previous bio Celia Winchell, M.D. Food and Drug Administration See previous bio Silvana Borges, M.D. Food and Drug Administration See previous bio Phillip Kronstein, M.D. Food and Drug Administration, CDER Phillip Kronstein, M.D., is a Senior Medical Officer in the Division of Psychiatry Products (DPP) at the U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER). In this position, Dr. Kronstein manages clinical reviews of Investigational New Drugs (INDs) and New Drug Applications (NDAs). Prior to joining the FDA in January 2008, he was a Clinical Research Fellow in the Experimental Therapeutics and Pathophysiology Branch at the National Institute of Mental Health, where he conducted trials in treatment-resistant depression and bipolar disorder. He received a Bachelor’s of Science in Chemistry from the University of Chicago in 1995 and a Doctor of Medicine from Tufts University School of Medicine in 2001. He completed his residency training in Psychiatry at the Johns Hopkins Hospital in June 2005. In addition to his review responsibilities at the FDA, Dr. Kronstein has been involved in regulatory research, looking at sexual dysfunction with antidepressants. He is also the Division Data Standards Lead for DPP as CDER, as part of a larger FDA initiative, continues to develop and implement standards to represent study data submitted in support of regulatory applications. 14 Acknowledgements Steering Committee Chairs Husseini Manji, M.D., FRCPC Michael E. Thase, M.D. Program Chairs Carlos Zarate, M.D. Holly A. Swartz, M.D. New Investigator Award Program Chairs Lauren D. Hill, Ph.D. Mark H. Rapaport, M.D. 15 Acknowledgements Acknowledgements Steering Committee Members Karl Broich, M.D. Federal Institute of Drugs and Medical Devices «Lori Davis, M.D. Tuscaloosa VA Medical Center Maurizio Fava, M.D. The Massachusetts General Hospital (ASCP Board Member) Marlene Freeman, M.D. Harvard Medical School (ASCP Board Member) Bruce Kinon, M.D. Eli Lilly & Company James H. Kocsis, M.D. Weill-Cornell Medical College David J. Kupfer, M.D. University of Pittsburgh School of Medicine Thomas P. Laughren, M.D. Food and Drug Administration Raye Litten, Ph.D. National Institute of Alcohol Abuse and Alcoholism «Anil K. Malhotra, M.D. Hofstra NS-LIJ School of Medicine The Zucker Hillside Hospital Husseini K. Manji, M.D., FRCPC Johnson & Johnson Pharmaceutical Research & Development Steve Marder, M.D. University of California Los Angeles David Michelson, M.D. Merck & Company, Inc. Andrew A. Nierenberg, M.D. Massachusetts General Hospital William Z. Potter, M.D., Ph.D. Foundation for the National Institute of Health Stephanie O’Malley, Ph.D. Yale University School of Medicine «New Investigator Alumni s Representing 16 ASCP CME Committee Acknowledgements Steering Committee Members (continued) s Mark H. Rapaport, M.D. Emory University School of Medicine (ASCP Board Member) Bob A. Rappaport, M.D. Center for Drug Evaluation and Research, FDA Steve Romano, M.D. Pfizer, Inc. Nina R. Schooler, Ph.D. State University of New York, Downstate Medical Center Philip Skolnick, Ph.D., D.Sc. National Institute on Drug Abuse Michael Thase, M.D. University of Pennsylvania School of Medicine (ASCP Board Member) Ben Vitiello, M.D. National Institute of Mental Health «Carlos A. Zarate, M.D. National Institute of Mental Health Program Committee Leslie Citrome, M.D., M.P.H. New York Medical College (ASCP Board Member) «Christoph U. Correll, M.D. The Zucker Hillside Hospital (ASCP Board Member) Thilo Deckersbach, Ph.D. Massachusetts General Hospital Bryan L. Dirks, M.D. Shire Pharmaceuticals Eden Evins, M.D. Harvard Medical School «Tiffany R. Farchione, M.D. Food and Drug Administration Maurizio Fava, M.D. Massachusetts General Hospital (ASCP Board Member) Bradley Gaynes, M.D. University of North Carolina «New Investigator Alumni s Representing 17 ASCP CME Committee Acknowledgements Program Committee (continued) John Greist, M.D. Healthcare Technology Systems, Inc. Richard Keefe, Ph.D. Duke University Medical Center Terence Ketter, M.D. Stanford University School of Medicine Helena Kraemer, Ph.D. Stanford University Charlotte Kremer, M.D. Astellas Pharmaceuticals David J. Kupfer, M.D. University of Pittsburgh School of Medicine Thomas P. Laughren, M.D. Food and Drug Administration «Anil Malhotra, M.D. Hofstra NS-LIJ School of Medicine, The Zucker Hillside Hospital Stephen Marder, M.D. Semel Institute, UCLA s Craig Nelson, M.D. University of California San Francisco «Katharine Phillips, M.D. Rhode Island Hospital/Brown University Steve Romano, M.D. Pfizer, Inc. Jerrold Rosenbaum, M.D. Massachusetts General Hospital Neil Ryan, M.D. University of Pittsburgh School of Medicine Martha Sajatovic, M.D. University Hospitals Case Medical Center Bruce Saltz, M.D., P.A. Mental Health Advocates, Inc. Richard Shelton, M.D. University of Alabama at Birmingham «New Investigator Alumni s Representing 18 ASCP CME Committee Acknowledgements Program Committee (continued) «Holly A. Swartz, M.D. University of Pittsburgh School of Medicine Benedetto Vitiello, M.D. National Institute of Mental Health Karen Wagner, M.D. University of Texas, Galveston (ASCP Board Member) «Janet Williams, D.S.W. MedAvante «Kimberly Yonkers, M.D. Yale School of Medicine «Carlos A. Zarate, M.D. National Institute of Mental Health New Investigator Award Committee «Christoph U. Correll, M.D. The Zucker Hillside Hospital «Lori Davis, M.D. Tuscaloosa VA Medical Center «Tiffany Farchione, M.D. Food and Drug Administration Lindsey Grandison, Ph.D. National Institute on Alcohol Abuse and Alcoholism Lauren D. Hill, Ph.D. National Institute of Mental Health Bruce Kinon, M.D. Eli Lilly & Company Ivan Montoya, M.D., MPH National Institute on Drug Abuse «Katharine Phillips, M.D. Rhode Island Hospital/Brown University s Mark H. Rapaport, M.D. Emory University School of Medicine (ASCP Board Member) Nina R. Schooler, Ph.D. State University of New York, Downstate Medical Center «Holly A. Swartz, M.D. University of Pittsburgh School of Medicine «New Investigator Alumni s Representing 19 ASCP CME Committee Acknowledgements ASCP Officers and Board Maurizio Fava, M.D. – ASCP President Massachusetts General Hospital s Mark H. Rapaport, M.D. – ASCP Treasurer Emory University School of Medicine Leslie Citrome, M.D., M.P.H. New York Medical College «Christoph U. Correll, M.D. The Zucker Hillside Hospital «Kristina Deligiannidis, M.D. University of Massachusetts Medical School Marlene Freeman, M.D. Massachusetts General Hospital Alan Gelenberg, M.D. Penn State University Ira Glick, M.D. Stanford University School of Medicine s Joseph Goldberg, M.D. Mount Sinai School of Medicine John Kane, M.D. The Zucker Hillside Hospital Arif Khan, M.D. Duke University School of Medicine John Newcomer, M.D. Leonard M. Miller School of Medicine, University of Miami Michael Thase, M.D. University of Pennsylvania Karen Wagner, M.D., Ph.D. University of Texas Medical Branch Sidney Zisook, M.D. University of California, San Diego «New Investigator Alumni s Representing 20 ASCP CME Committee Meeting Announcements Meeting Services Registration Desk Hours: Sunday 10:00 am – 5:00 pm Monday 7:30 am – 6:30 pm Tuesday 7:30 am – 6:45 pm Wednesday 7:30 am – 6:00 pm Thursday 7:30 am – 12:00 pm *The registration/meeting information desk is located at the main entrance of the Grand Ballroom. The Computer Center is open on the below dates and times for attendees to briefly check emails. The Computer Center is located in the Foyer of Diplomat 1-2. Hours: Sunday 12:00 pm – 5:00 pm Monday 8:00 am – 6:00 pm Tuesday – Wednesday 7:30 am – 6:30 pm Thursday 7:30 am – 12:00 pm Americans with Disabilities Act - It is the policy of ASCP not to discriminate against any person on the basis of disabilities. If you feel you need services or auxiliary aids mentioned in this act in order to fully participate in this continuing education activity, please call the Executive Office at 615-649-3085 or send an email to info@ascpp.org. Job Announcements may be posted on a message board at the ASCP registration desk. Discounts – All ASCP Annual Meeting attendees who booked their room at the Westin Diplomat through the ASCP meeting website will have the following resort amenities. • Access to Standard high-speed internet in the guest rooms for $6 per day • Fitness Center admittance • Daily newspaper • In-room coffee and tea 21 Meeting Announcements The ASCP Speaker Ready Room is open on the below dates and times for presenters to upload slides. The meeting organizers ask that all speakers upload their slides 24 hours prior to their scheduled presentation time. The Speaker Ready Room is located in Diplomat 3. Hours: Sunday 12:00 pm – 5:00 pm Monday 8:00 am – 6:00 pm Tuesday – Wednesday 7:30 am – 6:30 pm Thursday 7:30 am – 12:00 pm Meeting Announcements Continuing Education Credits Disclosures are available for all ASCP Annual Meeting presenters online at www.ASCPMeeting.org. Continuing Education Credits are available for physicians, pharmacists, psychologists and social workers. Applications for credit must be completed online with the meeting evaluation survey. The survey may be completed in the ASCP Computer Center in the Foyer of Diplomat 1-2 or after the conference at www.ASCPMeeting.org. Surveys for continuing education credit must be submitted no later than July 18, 2014. There is a $40.00 administrative fee for CME/CE applications. It is the policy of the ASCP to require disclosure of financial relationships from individuals in a position to control the content of a CME activity; to identify and resolve conflicts of interest related to those relationships; and to make disclosure information available to the audience prior to the CME activity. Presenters are required to disclose discussions of unlabeled/unapproved uses of drugs or devices during their presentations. Physicians The American Society for Clinical Psychopharmacology (ASCP) designates this live meeting for a maximum of 21.5 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity. Pharmacists USF Health is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. This knowledge-based program has been approved for 21.5 contact hours. Universal program number is as follows: 0230-9999-14-0130-L01-P. To receive continuing education credit, a pharmacist must attend the accredited sessions, actively participate in questions and answers and must return the program evaluation instrument. In order to receive full credit, registrants must arrive no later than 10 minutes after the start of the meeting and must attend the entire meeting. 22 Meeting Announcements Psychologists USF Health is approved by the American Psychological Association to sponsor continuing education for psychologists. USF Health maintains responsibility for this program and its content. This activity has been approved for 21.5 CE credits. Full attendance of the live activity is required. Partial credit will not be awarded. Social Workers USF Health is an approved provider (BAP #433 – exp. 3/31/15) of continuing education credits for clinical social work, marriage and family therapy, and mental health counseling. This program has been reviewed and approved for 25.8 credit hours, 50-minute contact hours. All participants who request continuing education credits by July 18, 2014, should expect to receive their statement of credits via email in late August. The Meeting Evaluation Survey will be available at www.ASCPMeeting.org. We encourage all registrants to complete the evaluation. Attendees requesting CME or CE credits must complete the survey in order to obtain credits. There is a $40.00 administrative fee for CME/CE applications. Your candid input on the 2014 meeting is appreciated as we strive to improve the meeting each year. ASCP Meeting Support – The 2014 ASCP Annual Meeting receives no corporate funding. 2015 ASCP Annual Meeting The 2015 ASCP Annual Meeting will take place June 22-25, 2015 at the Loews Miami Beach Hotel in Miami, Florida. Details regarding abstract submission for the 2015 Meeting will be released in September, 2014. 23 Notes 24 Sunday, June 15, 2014 8:30am – 4:30pm Co-Chairs: New Investigator Workshop (Invitation Only) Regency 1 Mark H. Rapaport, M.D., Emory University School of Medicine Lauren D. Hill, Ph.D., National Institute of Mental Health The ASCP Annual Meeting offers a special program for New Investigators in an effort to promote the education and training of junior investigators in psychopharmacology. Established investigators were asked to nominate individuals who may be interested in a research career in psychopharmacology for this special program. These nominees submitted an abstract describing their current research or area of research interest, a letter of recommendation from their chair or mentor, a career statement and a curriculum vitae. The selection of awardees was based upon the scientific merit of the abstract, the level of training of the nominee and a committee of internal and external reviewers’ assessment of the relative value of the specialized program to each applicant at this time in his/her career. The awardees will participate in this special educational workshop and present their posters during the scheduled poster sessions. In addition, they will receive a travel expense award and a certificate acknowledging their participation in the program at an award ceremony on Monday evening, June 16, 2014. This year’s 20 New Investigator awardees are indicated with a ribbon in the poster section of this program. Faculty Katharine Phillips, M.D. Rhode Island Hospital/Brown University Christoph U. Correll, M.D. The Zucker Hillside Hospital A. John Rush, M.D. Duke-National University of Singapore Lori Davis, M.D. Tuscaloosa VA Medical Center Nina R. Schooler, Ph.D. State University of New York, Downstate Medical Center Lindsey Grandison, Ph.D. National Institute on Alcohol Abuse and Alcoholism Lauren Hill, Ph.D. – Chair National Institute of Mental Health Holly Swartz, M.D. University of Pittsburgh School of Medicine Tiffany Farchione, M.D. Food and Drug Administration Special thanks to the ASCP New Investigator Program supporters: • Otsuka Pharmaceuticals, Inc. • Pharmaceutical Product Development (PPD) 25 Sunday Mark Bunker, Pharm.D. Cyberonics, Inc. Sunday, June 15, 2014 2014 New Investigator Awardees Eric Achytes, M.D., M.S. Michigan State University College of Human Medicine Kamilla Miskowiak, Ph.D. Psychiatric Center Copenhagen, Copenhagen University Hospital Ana Andreazza, Ph.D. University of Toronto, Departments of Pharmacology and Psychiatry Mark Niciu, M.D., Ph.D. National Institutes of Health (NIH)/ National Institute of Mental Health (NIMH) Shinichiro Nakajima, M.D., Ph.D. Centre for Addiction and Mental Health Hidehiro Oshibuchi, M.D., Ph.D. Department of Psychiatry, University of California, San Francisco Chaya Bhuvaneswaran, M.D., MPH Department of Psychiatry, University of Massachusetts-Worcester Kyle Burghardt, Pharm.D. University of Michigan College of Pharmacy M. Mercedes Perez-Rodriguez, M.D., Ph.D. Icahn School of Medicine at Mount Sinai and MIRECC at James J Peters VAMC Joan Camprodon, M.D., Ph.D., MPH Massachusetts General Hospital, Harvard Medical School Donna Roybal, M.D. University of Texas Health Sciences Center at San Antonio Michael Davis, M.D., Ph.D. VA Greater Los Angeles; University of California Los Angeles Diana Simeonova, Dipl. Psych., Ph.D. Emory University School of Medicine Stephanie Duhoux, Ph.D. Icahn School of Medicine at Mount Sinai Nhi-Ha Trinh, M.D., MPH Depression Clinical and Research Program, Massachusetts General Hospital Jennifer Felger, Ph.D. Emory University School of Medicine Gwyneth Zai, M.D., FRCPC, MSc Neurogenetics Section and Department of Psychiatry, Centre for Addiction and Mental Health, University of Toronto Philip Gerretsen, M.D., MSW Centre for Addiction and Mental Health Aaron Koenig, M.D. Western Psychiatric Institute & Clinic Clement Zai, Ph.D. Centre for Addiction and Mental Health 26 Monday, June 16, 2014 AT-A-GLANGE Monday, June 16, 2014 7:30am – 8:30am NIA Breakfast Roundtable (Invitation only) Room 216 8:30am – 9:00am Conference Opening Grand Ballroom 9:00am – 10:30am Panel Sessions *Recognizing and Treating Catatonia across the Diagnostic Spectrum: The Impact of New DSM-5 Classification Risks Posed by Duplicate or Inappropriate Subjects in Clinical Trials and Methods for Mitigating the Risk Research Forum: Meditative Practices, Underlying Neurobiological Mechanisms, and Application to Mental Health *Biological Approaches To Treat Substances Use Disorders Location: Diplomat 1-2 Location: Atlantic 1 Location: Atlantic 2 Location: Atlantic 3 10:30am – 10:45am Break Ballroom Foyers 10:45am – 12:15pm Panel Sessions *Novel Treatments in Bipolar Disorder: Primary and Secondary Targets Dimensional Symptom and Disability Measures in DSM-5 *Weighing In on Relative Risks of Fetal Exposure to Psychotropics and Psychiatric Disorders Location: Diplomat 1-2 Location: Atlantic 1 Location: Atlantic 2 Location: Atlantic 3 *of special interest to clinicians 27 Monday NIAAA Sponsored ACTIVE Update: Missing Data in Alcohol Use Disorder Clinical Trials - Issues and Analytic Methods Monday, June 16, 2014 12:15pm – 2:00pm Lunch On Own 1:00pm – 2:00pm First Time Attendee Meet & Greet Atlantic 1 2:00pm – 4:00pm Pharmaceutical Pipeline Presentations Grand Ballroom 4:00pm – 4:15pm Break Ballroom Foyers 4:15pm – 5:45pm Panel Sessions Placebo Response, Response Variance and Antidepressantplacebo Differences in Recent Antidepressant Clinical Trials based on Three Patient Interview Models *Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): A Pragmatic Trial of Lithium vs. a Second Generation Antipsychotic for Bipolar Disorder *NIAAA Panel Session: Advances in Treatments for PTSD and Alcohol Comorbidity Novel and Underutilized Strategies to Improve Adherence and Reduce Relapse Risk in Schizophrenia Location: Diplomat 1-2 Location: Atlantic 1 Location: Atlantic 2 Location: Atlantic 3 6:15pm – 7:45pm New Investigators’ Award Ceremony & Reception (Invitation only) Diplomat 4-5 *of special interest to clinicians 28 Monday, June 16, 2014 FULL SCHEDULE Monday, June 16, 2014 7:30am – 8:30am NIA Breakfast Roundtable (Invitation only) Room 216 8:30am – 9:00am Conference Opening Grand Ballroom Panel Sessions 9:00am – 10:30am *Recognizing and Treating Catatonia across the Diagnostic Spectrum: The Impact of New DSM-5 Classification Diplomat 1-2 Chair & Discussant: Georgios Petrides, The Zucker Hillside Hospital 9:00am – 9:10am Introduction 9:10am – 9:30am Catatonia: Video Workshop on Recognition and Management Andrew Francis, SUNY Stony Brook 9:30am – 9:50am Pediatric Catatonia: Review and New Vagal Theory Dirk M. Dhossche, University of Mississippi Medical Center 9:50am – 10:10am Longitudinal Assessment of the Psychomotor Dimension in Psychosis: Implications for Treatment Stanley N. Caroff, Philadelphia VA Medical Center/ University of Pennsylvania 10:10am – 10:30am Discussion *of special interest to clinicians 29 Monday, June 16, 2014 9:00am – 10:30am Risks Posed by Duplicate or Inappropriate Subjects in Clinical Trials and Methods for Mitigating the Risk Atlantic 1 Chair: Jonathan Rabinowitz, Bar Ilan University Discussant: Janet Williams, MedAvante, Inc. 9:00am – 9:10am Introduction 9:10am – 9:30am Evidence and Risks of Duplicate Subjects in Clinical Trials and How You Can Minimize the Risk Jonathan Rabinowitz, Bar Ilan University 9:30am – 9:50am The Professional Patient ‘Spectrum’ or Simply Inappropriate Patients: 50 Shades of Grey in Protocol Non-adherence Michael Detke, Indiana University Proven Strategies to Mitigate the Risk of Enrolling Professional Subjects in Large Depression Studies Brooke Geibel, Shire 10:10am – 10:30am Discussion 9:00am – 10:30am Research Forum: Meditative Practices, Underlying Neurobiological Mechanisms, and Application to Mental Health Atlantic 2 9:50am – 10:10am Chair: Emmeline Edwards, NIH/NCCAM Co-chair: Kristen Huntley, NIH/NCCAM Discussant: David Shurtleff, NIH/NCCAM 9:00am – 9:10am Introduction 9:10am – 9:30am Neuroimaging as a Tool for the Study of Meditation Kelvin O. Lim, University of Minnesota 9:30am – 9:50am What is Known about the Effectiveness of Meditative Approaches for Resilience and Cognitive Enhancement? Amishi Jha, University of Miami 30 Monday, June 16, 2014 9:00am – 10:30am (continued) Research Forum: Meditative Practices, Underlying Neurobiological Mechanisms, and Application to Mental Health Atlantic 2 9:50am – 10:10am Innovation and Opportunities in Neuroscientific Research on Meditation Richard J. Davidson, University of Wisconsin 10:10am – 10:30am Discussion 9:00am – 10:30am *Biological Approaches to Treat Substances Use Disorders Atlantic 3 Chair: Phil Skolnick, NIDA/NIH Discussant: Raye Litten, NIAAA 9:00am – 9:10am Introduction 9:10am – 9:25am Genetically Engineered Butyrylcholinesterase (TV-1380): An Innovative Approach to Treat Cocaine Dependence Phil Skolnick, NIDA/NIH 9:25am – 9:40am Use of Functional Assays to Develop a Novel Antinicotine Vaccine Heather L. Davis, Pfizer Vaccine Immunotherapeutics 9:40am – 9:55am Development of a Heroin Vaccine Gary R. Matyas, Walter Reed Army Institute of Research 9:55am – 10:10am Continuing Towards Gene Transfer of Modified Human Butyrylcholinesterase to Treat Cocaine Addiction Stephen Brimijoin, Mayo Clinic 10:10am – 10:30am Discussion 10:30am – 10:45am Break Ballroom Foyers *of special interest to clinicians 31 Monday, June 16, 2014 Panel Sessions 10:45am – 12:15pm NIAAA Sponsored ACTIVE Update: Missing Data in Alcohol Use Disorder Clinical Trials - Issues and Analytic Methods Diplomat 1-2 Chair: Raymond F. Anton, Medical University of South Carolina Discussant: Raye Z. Litten, NIAAA 10:45am – 10:55am Introduction 10:55am – 11:10am Overview of the ACTIVE Workgroup and Mission and Importance of Missing Drinking Data in Alcohol Use Disorder Trials Raymond F. Anton, Medical University of South Carolina 11:10am – 11:25am 11:25am – 11:40am How Does Drinking Change When People Stop Taking Medicines During a Clinical Trial? Implications for Missing Drinking Data Reduction and Imputation Robert L. Stout, Pacific Institute for Research and Evaluation The Use of Pattern Mixture Models for Imputation and Analysis of Missing Drinking Data in Nalmfene Trials for Alcohol Dependence During Regulatory Approval in Europe Per Sorensen, H. Lundbeck A/S Validity of Various Missing Drinking Data Imputation Methods – Results from Re-analysis of the US COMBINE Study Katie Witkiewitz, University of New Mexico 11:55am – 12:15pm Discussion 11:40am – 11:55am 32 Monday, June 16, 2014 10:45am – 12:15pm *Novel Treatments in Bipolar Disorder: Primary and Secondary Targets Atlantic 1 Chair: Katherine Burdick, Mount Sinai School of Medicine Discussant: Terence Ketter, Stanford University School of Medicine 10:45am – 10:55am Introduction 10:55am – 11:10am Methodological Considerations in the Design and Conduct of Acute Adjunctive Bipolar Depression Treatment Trials Joseph R. Calabrese, Case Western Reserve School of Medicine 11:10am – 11:25am 11:25am – 11:40am 11:40am – 11:55am Effect of Lurasidone on Cognitive Impairment: From the Lab to the Clinic Andrei Pikalov, Sunovion Pharmaceuticals, Inc. Methodological Challenges to Cognitive Trials in Bipolar Disorder Katherine Burdick, Mount Sinai School of Medicine Interaction Effects between Affective Symptoms and Cognitive Function in Bipolar Disorder Clinical Trials Joseph F. Goldberg, Icahn School of Medicine at Mount Sinai 11:55am – 12:15pm Discussion 10:45am – 12:15pm Dimensional Symptom and Disability Measures in DSM-5 Atlantic 2 Chair: William Narrow, American Psychiatric Association Discussant: Lori Davis, Tuscaloosa VA Medical Center and University of Alabama School of Medicine 10:45am – 10:55am Introduction 10:55am – 11:15am DSM-5 Cross-cutting Dimensional Measures: Reliability, Sensitivity to Change, and Association with Disability Diana E. Clarke, American Psychiatric Association *of special interest to clinicians 33 Monday, June 16, 2014 10:45am – 12:15pm Dimensional Symptom and Disability Measures in (continued)DSM-5 Atlantic 2 11:15am – 11:35am DSM-5 Dimensional Symptom and Disability Measures in Routine Clinical Practice Settings Eve K. Mościcki, American Psychiatric Association The World Health Organization Disability Assessment Schedule in the DSM-5 Field Trials: Associations with Psychiatric Diagnosis William Narrow, American Psychiatric Association 11:55am – 12:15pm Discussion 10:45am – 12:15pm *Weighing In on Relative Risks of Fetal Exposure to Psychotropics and Psychiatric Disorders Atlantic 3 11:35am – 11:55am Chair: Lee S. Cohen, Massachusetts General Hospital Co-chair & Discussant: Marlene Freeman, Massachusetts General Hospital 10:45am – 10:55am Introduction 10:55am – 11:15am The National Pregnancy Registry for Atypical Antipsychotics: Effects of Fetal Exposure on Risk for Congenital Malformations and Maternal and Newborn Outcomes Lee S. Cohen, Massachusetts General Hospital 11:15am – 11:35am 11:35am – 11:55am Prenatal Exposure to SSRIs: Sorting the Evergrowing Data Sonia Hernandez-Diaz, Harvard School of Public Health Impact of Maternal Psychiatric Illness on Fetal, Obstetrical and Neonatal Wellbeing Margaret Altemus, Weill Medical College, Cornell University 11:55am – 12:15pm Discussion 12:15pm – 2:00pm Lunch On Own 1:00pm – 2:00pm First Time Attendee Meet & Greet Atlantic 1 *of special interest to clinicians 34 Monday, June 16, 2014 2:00pm – 4:00pm Pharmaceutical Pipeline Presentations Grand Ballroom Chair: Carlos Zarate, National Institute of Mental Health 2:00pm – 2:10pm 2:10pm – 2:20pm 2:20pm – 2:30pm 2:30pm – 2:40pm 2:40pm – 2:50pm 2:50pm – 3:00pm 3:00pm – 3:10pm A Pilot Study of a Novel Monoamine Triple Reuptake Inhibitor EB-1020 SR in the Treatment of ADHD in Adults Timothy Wilens, Massachusetts General Hospital Metadoxine Extended Release (MDX): A Novel Drug Candidate for the Treatment of ADHD & Other Cognitive Disorders Jonathan Rubin, Alcobra Pharma A Rapidly Acting Intranasal Treatment for the Symptoms of GAD Michael R. Liebowitz, Pherin Pharmaceuticals Lupron in Combination with an Acetylcholinesterase Inhibitor Halts Cognitive Decline in Women with Alzheimer’s Disease over a 48 Week Period Richard Bowen, OTB Research Efficacy and Safety of a Novel mGlu2 Receptor Positive Allosteric Modulator as an Adjunctive Treatment to an SSRI/SNRI in the Treatment of Anxious Depression Justine Kent, Janssen A Double-blind, Randomized, Placebo-controlled, Parallel Group, Dose Frequency Study of Intravenous Ketamine in Patients with Treatmentresistant Depression Jaskaran Singh, Janssen Research and Development, LLC Randomized, Double-blind, Active-controlled, Phase 2/3 Study to Determine the Short-term (6-Week) and Long-term (6 Month) Cognitive and Anti-psychotic Efficacy, Safety and Tolerability of CYP-1020 Compared to Risperidone Jonathan Rabinowitz, Bar Ilan University 35 Monday, June 16, 2014 2:00pm – 4:00pm (continued) Pharmaceutical Pipeline Presentations Grand Ballroom 3:10pm – 3:20pm Results of a Phase 2B Clinical Trial of TC-5619, a Selective Alpha 7 Neuronal Nicotinic Receptor (NNR) Agonist in the Adjunctive Treatment of Negative Symptoms and Cognitive Dysfunction in Schizophrenia David Hosford, Targacept, Inc. 3:20pm – 3:30pm AZD8529, a Positive Allosteric Modulator of the mGluR2 Receptor for the Treatment of Schizophrenia Alan Cross, AstraZeneca Neuroscience Innovative Medicines Unit Advancing ITI-007: A Novel Product Candidate for the Treatment of Schizophrenia, Bipolar Disorder and Other Neuropsychiatric Indications Kimberly E. Vanover, Intra-Cellular Therapies, Inc. 4:00pm – 4:15pm Break Ballroom Foyers 3:30pm – 3:40pm Panel Sessions 4:15pm – 5:45pm Placebo Response, Response Variance and Antidepressant-placebo Differences in Recent Antidepressant Clinical Trials Based on Three Patient Interview Models Diplomat 1-2 Chair: Arif Khan, Northwest Clinical Research Center Discussant: Walter Brown, Brown University 4:15pm – 4:25pm Introduction 4:25pm – 4:45pm Examining the Utility and Futility of Surveillance Strategies for CNS Trials Steven D. Targum, Clintara, LLC 36 Monday, June 16, 2014 4:15pm – 5:45pm (continued) Placebo Response, Response Variance and Antidepressant-placebo Differences in Recent Antidepressant Clinical Trials Based on Three Patient Interview Models Diplomat 1-2 4:45pm – 5:05pm The Use of Blinded, Independent, Remote Ratings in Psychiatric Clinical Trials: The Good, the Bad, and the Appropriate Situation Michael Detke, Indiana University Magnitude of Placebo Response and Response Variance in Antidepressant Clinical Trials using Enhanced Interviews Techniques Compared to Traditional Rating Interviews Arif Khan, Northwest Clinical Research Center 5:25pm – 5:45pm Discussion 4:15pm – 5:45pm *Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): A Pragmatic Trial of Lithium vs. a Second Generation Antipsychotic for Bipolar Disorder Atlantic 1 5:05pm – 5:25pm Chair: Terence Ketter, Stanford University School of Medicine Co-chair: Andrew Nierenberg, Massachusetts General Hospital Discussant: Mauricio Tohen, University of New Mexico 4:15pm – 4:25pm Introduction 4:25pm – 4:45pm 4:45pm – 5:05pm Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): Rationale, Design, and Demographics Edward S. Friedman, University of Pittsburgh School of Medicine Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness) Andrew Nierenberg, Massachusetts General Hospital *of special interest to clinicians 37 Monday, June 16, 2014 4:15pm – 5:45pm (continued) *Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): A Pragmatic Trial of Lithium vs. a Second Generation Antipsychotic for Bipolar Disorder Atlantic 1 5:05pm – 5:25pm Bipolar CHOICE Safety and Tolerability Outcomes: Focus on Obesity and Cardiometabolic Health David Kemp, Case Western Reserve University 5:25pm – 5:45pm Discussion 4:15pm – 5:45pm *NIAAA Panel Session: Advances in Treatments for PTSD and Alcohol Comorbidity Atlantic 2 Chair: Raye Z. Litten, NIAAA Co-chair: Ismene L. Petrakis, Yale University School of Medicine Discussant: Raye Z. Litten, NIAAA 4:15pm – 4:25pm Introduction 4:25pm – 4:45pm Pharmacotherapy of Patients with Post Traumatic Stress Disorder (PTSD) and Comorbid Alcohol Use Disorders among Veterans Ismene L. Petrakis, Yale University School of Medicine 4:45pm – 5:05pm Prazosin for Comorbid PTSD and Alcohol Dependence: A Pilot Randomized Clinical Trial Tracy Simpson, VA Puget Sound Health Care System Effective Treatment Strategies for Patients with Concurrent PTSD and Addiction David Oslin, University of Pennsylvania 5:25pm – 5:45pm Discussion 5:05pm – 5:25pm *of special interest to clinicians 38 Monday, June 16, 2014 4:15pm – 5:45pm Novel and Underutilized Strategies to Improve Adherence and Reduce Relapse Risk in Schizophrenia Atlantic 3 Chair: Christoph Correll, The Zucker Hillside Hospital Discussant: Nina R. Schooler, SUNY Downstate Medical Center 4:15pm – 4:25pm Introduction 4:25pm – 4:40pm Response, Remission and Recovery in Schizophrenia John M. Kane, The Zucker Hillside Hospital 4:40pm – 4:55pm 4:55pm – 5:10pm Effect of Trial Design, Population and Illness Phase on the Role of Long-acting Injectable Antipsychotics for Signaling and Preventing Non-adherence in Schizophrenia Christoph Correll, The Zucker Hillside Hospital Leveraging Novel Technologies to Enhance Adherence Adam Hanina, Ai Cure Technologies Technology-based Approaches for the Detection and Prevention of Relapse in Schizophrenia Dror Ben-Zeev, Dartmouth College 5:25pm – 5:45pm Discussion 6:15pm – 7:45pm New Investigators’ Award Ceremony & Reception (Invitation only) Diplomat 4-5 5:10pm – 5:25pm 39 Notes 40 Tuesday, June 17, 2014 Tuesday AT-A-GLANCE Tuesday, June 17, 2014 6:45am – 8:00am 15th Annual Fun Run/Walk Meet in Main Lobby 7:00am – 8:30am ASCP Board Meeting (Invitation only) Room 217 7:30am – 8:30am NIA Breakfast Roundtable (Invitation only) Room 216 7:30am – 9:00am Morning Break Ballroom Foyers 8:30am – 10:00am Regulatory Plenary: FDA Science Initiatives: A Brief Update Grand Ballroom 10:00am – 10:15amBreak Ballroom Foyers ASCP Lifetime Awardee Presentation – A. John Rush: Bridging the Chasm between Research and Practice Grand Ballroom 11:15am – 1:00pm Poster Session I with Lunch Regency Ballroom 1:15pm – 2:45pm Panel Sessions 10:15am – 11:15am *Overcoming the Shortcomings of Treatment Practice Guidelines for Mood and Psychotic Disorders NIMH/NCCAM Panel: Conducting Pragmatic Trials in Mental Health: Lessons Learned from the NIH Health Care Systems Research Collaboratory Inflammation and Insulin Resistance: Implications for Pathophysiology and Treatment *Long Acting Injectable Antipsychotics: Perspectives on their Role in Schizophrenia Treatment Location: Diplomat 1-2 Location: Atlantic 1 Location: Atlantic 2 Location: Atlantic 3 *of special interest to clinicians 41 Tuesday, June 17, 2014 ASCP Business Meeting (ASCP Members Only) Grand Ballroom 2:45pm – 3:15pm 3:15pm – 3:30pmBreak Ballroom Foyers Individual Research Reports 3:30pm – 4:30pm Anxiety Disorder Presentations Depression Presentations Schizophrenia and Bipolar Disorder Presentations Statistical Methods, Personality Disorders, Substance Abuse, and Comorbidity Presentations Location: Diplomat 1-2 Location: Atlantic 1 Location: Atlantic 2 Location: Atlantic 3 4:30pm – 4:45pmBreak Ballroom Foyers 4:45pm – 6:45pm Workshops Cognitive Deficits in Depression: What are They? Are They Independent Dimensions? Are They Targets for Treatment? *Psychopharmacology of Residual Symptoms in Mood Disorders and Schizophrenia New Approaches to Funding Clinical Trials at NIMH Location: Atlantic 1 Location: Atlantic 2 Location: Atlantic 3 7:00pm – 8:00pm ASCP Reception South Palm Court 9:00pm – 12:00am Black & White Affair, hosted by Zane Courbay Room 3641 *of special interest to clinicians 42 Tuesday, June 17, 2014 FULL SCHEDULE Tuesday, June 17, 2014 6:45am – 8:00am 15th Annual Fun Run/Walk Meet in Main Lobby 7:00am – 8:30am ASCP Board Meeting (Invitation only) Room 217 7:30am – 8:30am NIA Breakfast Roundtable (Invitation only) Room 216 7:30am – 9:00am Morning Break Ballroom Foyers 8:30am – 10:00am Regulatory Plenary: FDA Science Initiatives: A Brief Update Grand Ballroom Chair: Ni A. Khin, M.D., U.S. Food and Drug Administration This session will provide updates on regulatory initiatives from the US Food and Drug Administration (FDA). Dr. Ni Khin will give a brief overview of the joint FDA initiative with European Medicines Agency (EMA) to ensure data quality in clinical trials and good clinical practice compliance. Dr. Celia Winchell from FDA’s Division of Anesthesia and Analgesia Products (DAAP) will discuss the challenges of determining efficacy endpoints in clinical trials for addiction treatment drugs. Specifically, she will discuss how DAAP identified a pattern of alcohol use as an alternative endpoint to complete abstinence based on recent analyses of data. Dr. Silvana Borges from FDA’s Division of Psychiatry Products will present preliminary findings regarding use of active controls in depression trials. There will be an informal discussion with the audience on these selected topics as well as other regulatory issues of common interest within this context. 8:30am – 8:45am Ni A. Khin, M.D., Food and Drug Administration 8:45am – 9:05am Celia Winchell, M.D., Food and Drug Administration 9:05am – 9:25am Silvana Borges, M.D., Food and Drug Administration 9:25am – 10:00am Panel Discussion and Q&A 43 Tuesday, June 17, 2014 10:00am – 10:15am Break Ballroom Foyers 10:15am – 11:15am ASCP Lifetime Awardee Presentation – A. John Rush: Bridging the Chasm between Research and Practice Grand Ballroom Patient centered research and comparative effectiveness research address practical issues that aim at addressing patients’ concerns or choices among treatments, respectively. In addition to these important objectives, clinicians need to better understand how to deliver each treatment; to whom to deliver (or not) particular treatments; when to discontinue, switch, or augment a specific treatment; and for whom which specific treatment sequences are indicated. This presentation discusses practical, simple, efficient research design, measurement and analytic options, that could address these important clinical knowledge gaps with the aim of improving patient outcomes and treatment cost efficiencies. 11:15am – 1:00pm Poster Session I with Lunch Regency Ballroom *See Pages 69 through 80 for poster listing. Panel Sessions 1:15pm – 2:45pm *Overcoming the Shortcomings of Treatment Practice Guidelines for Mood and Psychotic Disorders Diplomat 1-2 Chair: Joseph F. Goldberg, Icahn School of Medicine at Mount Sinai Discussant: Michael E. Thase, Perelman School of Medicine of the University of Pennsylvania 1:15pm – 1:25pm Introduction 1:25pm – 1:45pm Practice Guidelines for Bipolar Disorder: What’s Useful, What’s Not, and What’s Missing Joseph F. Goldberg, Icahn School of Medicine at Mount Sinai *of special interest to clinicians 44 Tuesday, June 17, 2014 1:15pm – 2:45pm (continued) *Overcoming the Shortcomings of Treatment Practice Guidelines for Mood and Psychotic Disorders Diplomat 1-2 1:45pm – 2:05pm Treatment Guidelines for MDD: Evidence-based, Eminence-based, or Faith-based? Alan J. Gelenberg, Penn State College of Medicine Practice Guidelines for Treatment of Schizophrenia: Consensus or Confusion? Peter J. Weiden, UIC Medical Center 2:25pm – 2:45pm Discussion 1:15pm – 2:45pm NIMH/NCCAM Panel: Conducting Pragmatic Trials in Mental Health: Lessons Learned from the NIH Health Care Systems Research Collaboratory Atlantic 1 2:05pm – 2:25pm Chair & Discussant: Emmeline Edwards, NIH, NCCAM Co-chair: Wendy Weber, NIH, NCCAM 1:15pm – 1:25pm Introduction 1:25pm – 1:45pm Conducting Pragmatic Trials in Mental Health: Lessons Learned from the NIH Health Care Systems Research Collaboratory Wendy Weber, NIH, NCCAM 1:45pm – 2:05pm Pragmatic Trial of Population-based Program to Prevent Suicide Attempts Greg Simon, Group Health Research Institute NIMH/NCCAM Panel: Conducting Pragmatic Trials in Mental Health: Lessons Learned from the NIH Health Care Systems Research Collaboratory Liz Delong, Duke University Medical Center 2:25pm – 2:45pm Discussion 2:05pm – 2:25pm *of special interest to clinicians 45 Tuesday, June 17, 2014 1:15pm – 2:45pm Inflammation and Insulin Resistance: Implications for Pathophysiology and Treatment Atlantic 2 Chair: David Kemp, Case Western Reserve University Discussant: Madhukar Trivedi, UT Southwestern 1:15pm – 1:20pm Introduction 1:20pm – 1:35pm PPAR-γ Agonism as a Modulator of Mood: Proofof-concept for Pioglitazone in Bipolar Depression David Kemp, Case Western Reserve University 1:35pm – 1:50pm 1:50pm – 2:05pm Association between Kynurenine-pathway Metabolites and Gray Matter Volumes of the Hippocampus and Amygdala in Patients with Mood Disorders Jonathan Savitz, Laureate Institute for Brain Research Trait and State Patterns of Inflammatory Biomarkers in Bipolar Disorder Jess G. Fiedorowicz, University of Iowa 2:05pm – 2:20pm Biomarkers of Illness Activity in Bipolar Disorder Flavio Kapczinski, Federal University of Rio Grande do Sul 2:20pm – 2:45pm Discussion 1:15pm – 2:45pm *Long Acting Injectable Antipsychotics: Perspectives on their Role in Schizophrenia Treatment Atlantic 3 Chair: Nina R. Schooler, SUNY Downstate Medical Center Discussant: John M. Kane, The Zucker Hillside Hospital 1:15pm – 1:25pm Introduction 1:25pm – 1:45pm Long Acting Injectable vs. Oral Antipsychotics for Schizophrenia: Meta-analytic Consideration of the True Effect Size by the Study Designs Taishiro Kishimoto, Keio University School of Medicine, Department of Psychiatry *of special interest to clinicians 46 Tuesday, June 17, 2014 1:15pm – 2:45pm (continued) *Long Acting Injectable Antipsychotics: Perspectives on their Role in Schizophrenia Treatment Atlantic 3 1:45pm – 2:05pm PROACTIVE: Exploring Longitudinal Course to Understand Treatment Outcomes in LAI - oral Comparisons Nina R. Schooler, SUNY Downstate Medical Center A Comparison of Long-acting Antipsychotic Medications for Schizophrenia (ACLAIMS) Joseph P. McEvoy, Georgia Regents University 2:25pm – 2:45pm Discussion 2:45pm – 3:15pm ASCP Business Meeting (ASCP Members Only) Grand Ballroom 3:15pm – 3:30pm Break Ballroom Foyers 2:05pm – 2:25pm Individual Research Reports 3:30pm – 4:30pm Anxiety Disorder Presentations Diplomat 1-2 Chair: Crystal Clark, Northwestern University Feinberg School of Medicine Asher Center for the Study and Treatment of Depressive Disorders 3:30pm – 3:45pm 3:45pm – 4:00pm A Rapidly Acting Intranasal Treatment for the Symptoms of GAD Michael R. Liebowitz, Pherin Pharmaceuticals Comparative Effectiveness of Prolonged Exposure (PE) and Sertraline(SER) in PTSD: Final Analyses of The Impact of Choice and Treatment Preference on Acute Outcome Matig Mavissakalian, Case Western Reserve University School of Medicine *of special interest to clinicians 47 Tuesday, June 17, 2014 3:30pm – 4:30pm (continued) Anxiety Disorder Presentations Diplomat 1-2 4:00pm – 4:15pm Pharmacogenetic Study of Genetic Variations across Remote Regulatory Regions of 14 Obsessive-Compulsive Disorder Candidate Genes in Antidepressant Response Gwyneth Zai, University of Toronto Emotion Recognition Deficits in Treated and Untreated Adults with ADHD Anneka Tomlinson, University of Manchester 3:30pm – 4:30pm Depression Presentations Atlantic 1 4:15pm – 4:30pm Chair: Bradley Gaynes, University of North Carolina School of Medicine 3:30pm – 3:45pm 3:45pm – 4:00pm 4:00pm – 4:15pm 4:15pm – 4:30pm The Efficacy of Vortioxetine in Patients with Major Depressive Disorder and High Levels of Anxiety Symptoms: A Meta-analysis David Baldwin, University of Southampton Erythropoietin Induces Growth in Left Hippocampus and Improves Verbal Memory in Patients with Severe Affective Disorders Kamilla Woznica Miskowiakm, Copenhagen University Hospital Epidural Cortical Stimulation of the Left DLPFC Leads to Dose-dependent Enhancement of Working Memory in Patients with MDD Joan A. Camprodon, Massachusetts General Hospital, Harvard Medical School A Phase 1B, Randomized, Double-blind, Placebocontrolled, Multiple-dose Escalation Study Evaluating the Effects of NSI-189 Phosphate, a Neurogenic Compound, in Patients with Major Depressive Disorder (MDD) Marlene Freeman, Massachusetts General Hospital = New Investigator 48 Tuesday, June 17, 2014 3:30pm – 4:30pm Schizophrenia and Bipolar Disorder Presentations Atlantic 2 Chair: Kristina Deligiannidis, University of Massachusetts Medical School/ UMass Memorial Medical Center 3:30pm – 3:45pm 3:45pm – 4:00pm 4:00pm – 4:15pm 4:15pm – 4:30pm 3:30pm – 4:30pm Can Oxytocin Enhance Learning during Social Cognitive Skills Training in Schizophrenia? Michael C. Davis, VA Greater Los Angeles, University of California Los Angeles Hospitalization Rates in Patients Switched from Oral Antipsychotics to Aripiprazole Once-monthly: A Mirror Study Timothy Peters-Strickland, Otsuka Pharmaceutical Development &Commercialization, Inc. Lithium Enhances Mitochondrial Complex I Activity and Ameliorates DNA Methylation and Hydroxymethylation Induced by Mitochondrial Complex I Dysfunction Ana Cristina Andreazza, University of Toronto Varenicline for Smoking Cessation in Bipolar Disorder: A Double-blind, Randomized, Placebocontrolled Trial Roy K.N. Chengappa, University of Pittsburgh Statistical Methods, Personality Disorders, Substance Abuse, and Comorbidity Presentations Atlantic 3 Chair: Katherine Burdick, Mount Sinai School of Medicine 3:30pm – 3:45pm 3:45pm – 4:00pm Brain-derived Neurotrophic Factor Genotype and Amygdala Habituation in Borderline Personality Disorder M. Mercedes Perez-Rodriguez, Mount Sinai School of Medicine Analysis and Missing Data Handling in Psychiatry Trials with Inevitable, High, Differential and Informative Discontinuations Yangchun Du, Alkermes, Inc. = New Investigator 49 Tuesday, June 17, 2014 3:30pm – 4:30pm (continued) Statistical Methods, Personality Disorders, Substance Abuse, and Comorbidity Presentations Atlantic 3 4:00pm – 4:15pm Long-term Skeletal Effects of Risperidone and SSRIs in Youths Chadi Calarge, University of Iowa Impulsivity and Substance Dependence: Metaanalysis and Possible Role in Treatment Saddichha Sahoo, NIMHANS 4:30pm – 4:45pm Break Ballroom Foyers 4:15pm – 4:30pm Workshops 4:45pm – 6:45pm Cognitive Deficits in Depression: What are they? Are they Independent Dimensions? Are they Targets for Treatment? Atlantic 1 Chair: Steven D. Targum, Clintara, LLC Co-chair: Craig Nelson, UCSF Discussant: Tiffany Farchione, US Food and Drug Administration 4:45pm – 4:50pm Introduction 4:50pm – 5:10pm Cognitive Impairment in Late Life Depression: Type, Frequency, and Methods of Assessment Scott Mackin, University of California, San Francisco 5:10pm – 5:30pm 5:30pm – 5:50pm 5:50pm – 6:10pm Effects of Treatment on Cognition in Late Life Depression Craig Nelson, UCSF Changes in Cognitive Symptoms before and after Buspirone-melatonin Treatment for Major Depressive Disorder Steven D. Targum, Clintara, LLC Changes in Cognitive Symptoms before and after Vortioxetine Treatment in Major Depressive Disorder Maurizio Fava, Massachusetts General Hospital 50 Tuesday, June 17, 2014 4:45pm – 6:45pm (continued) 6:10pm – 6:30pm Cognitive Deficits in Depression: What are they? Are they Independent Dimensions? Are they Targets for Treatment? Atlantic 1 Deficits in Mood Disorders: Impact on Functional Outcomes and Treatment Strategies Dan V. Iosifescu, Icahn School of Medicine at Mount Sinai 6:30pm – 6:45pm Discussion 4:45pm – 6:45pm *Psychopharmacology of Residual Symptoms in Mood Disorders and Schizophrenia Atlantic 2 Chair: Jonathan Alpert, Massachusetts General Hospital, Harvard Medical School Discussant: Richard Shelton, University of Alabama at Birmingham 4:45pm – 4:50pm Introduction 4:50pm – 5:15pm Methodological and Design Issues in Augmentation Trials Thomas Laughren, MGH CTNI 5:15pm – 5:40pm 5:40pm – 6:05pm 6:05pm – 6:30pm 6:30pm – 6:45pm New Approaches to the Treatment of Residual Symptoms of Schizophrenia Donald C. Goff, NYU Medical School Residual Symptoms in Bipolar Disorder: The Role of Polypharmacy Joseph F. Goldberg, Icahn School of Medicine at Mount Sinai Studying the Efficacy of Adjunctive Therapies for Depressive Disorders Michael E. Thase, Perelman School of Medicine of the University of Pennsylvania Discussion *of special interest to clinicians 51 Tuesday, June 17, 2014 4:45pm – 6:45pm New Approaches to Funding Clinical Trials at NIMH Atlantic 3 Chair: William Potter, National Institute of Mental Health Discussant: David Kupfer, University of Pittsburgh School of Medicine 4:45pm – 5:00pm Introduction 5:00pm – 5:20pm First in Human and Early Stage Clinical Trials of Novel Investigational Drugs or Devices for Psychiatric Disorders Meg Grabb, National Institute of Mental Health 5:20pm – 5:40pm Exploratory Clinical Trials of Novel Interventions for Mental Disorders Jill Heemskerk, National Institute of Mental Health Pilot Effectiveness Studies and Services Research Grants Chris Sarampote, National Institute of Mental Health 6:00pm – 6:45pm Discussion 7:00pm – 8:00pm ASCP Reception South Palm Court 5:40pm – 6:00pm 52 Wednesday, June 18, 2014 AT-A-GLANCE Wednesday, June 18, 2014 ASCP Steering Committee Meeting (Invitation only) Room 308 7:30am – 8:30am NIA Breakfast Roundtable (Invitation only) Room 216 7:30am – 9:00am Morning Break Ballroom Foyers 8:15am – 9:45am Keynote Session: New Approaches to Mental Illness in the Era of the National Brain Initiative Grand Ballroom 9:45am – 10:00am Break Ballroom Foyers 10:00am – 12:00pm Plenary Session – NIH Institute Directors Grand Ballroom 12:00pm – 2:00pm Poster Session II with Lunch Regency Ballroom 2:00pm – 3:30pm Updates Session – The Latest on Treatment of Mood, OCD-spectrum, and Binge Eating Disorders Grand Ballroom 3:30pm – 3:45pm Break Ballroom Foyers 3:45pm – 5:45pmWorkshops New Approaches to Drug Studies for Treating Social Deficits in Autism Spectrum Disorder *Novel Mechanisms of Action for the Treatment of Depression and Anxiety: Scientific Updates *Psychiatry and Technology: A Partnership in Promoting Mental Health Location: Atlantic 1 Location: Atlantic 2 Location: Atlantic 3 *of special interest to clinicians 53 Wednesday 7:00am – 8:30am Notes 54 Wednesday, June 18, 2014 FULL SCHEDULE Wednesday, June 18, 2014 7:00am – 8:30am ASCP Steering Committee Meeting (Invitation only) Room 308 7:30am – 8:30am NIA Breakfast Roundtable (Invitation only) Room 216 7:30am – 9:00am Morning Break Ballroom Foyers 8:15am – 9:45am Keynote Session: New Approaches to Mental Illness in the Era of the National Brain Initiative Grand Ballroom Chair: Husseini K. Manji, M.D., FRCPC, Johnson & Johnson Brain disorders are among mankind’s most devastating illnesses. Worldwide, they place an enormous societal burden on those affected. Indeed, in the United States alone this burden of illness is rapidly approaching $1 trillion annually, a number that is only likely to escalate in coming years with the aging population. In this plenary session, Drs. Manji and Insel and Mr. Kennedy will discuss interrelated facets in our search to better understand the mechanisms underlying a wide range of mental illnesses and to develop effective new treatments for them. Rapid advances in science and technology over the past decade have provided us with an unprecedented opportunity and the tools needed to unlock the secrets of the brain. Dr. Insel will discuss the many significant advances that have recently been made towards understanding serious mental illnesses. Although public and private resources devoted to research in this area are diminishing, a host of cutting-edge approaches— from genomics to data mining, proteomics to biomarkers, pathway modeling to protein engineering, neuroimaging to optogenetics—is nevertheless revolutionizing the way we think about, study, and approach the development of urgently needed novel treatments for mental disorders, with extremely promising results. 55 Wednesday, June 18, 2014 8:15am – 9:45am (continued) Keynote Session: New Approaches to Mental Illness in the Era of the National Brain Initiative Grand Ballroom Dr. Manji will discuss the paradigm shift that must accompany future research in this area. This includes not only moving from a ‘diagnose and treat’ approach to a ‘predict and pre-empt’ model, but the need to develop novel solutions that encompass meaningful and measurable patient outcomes (for instance, the ability to rapidly resume social and work responsibilities). Mr. Kennedy will discuss the many social issues that can and must be addressed in any “whole-world” view of mental illness, including parity for mental health, ending the discrimination against patients, and the travesty of homeless and imprisoned individuals suffering from mental disorders. The session will emphasize the speakers’ commitment to a strong, united, cross-disciplinary approach towards a key common goal: to work together across industry, academia, government, and the private sector in a concerted effort to improve the lives of the millions of individuals affected by brain disorders. With such a cooperative effort, real, tangible progress can be made. 8:15am – 8:25am Introduction Husseini K. Manji, M.D., FRCPC, Johnson & Johnson 8:25am – 8:55am Thomas Insel, M.D., NIMH 8:55am – 9:25am Patrick Kennedy, Former US Representative & Mental Health Activist 9:25am – 9:45am Discussion 9:45am – 10:00am Break Ballroom Foyers 56 Wednesday, June 18, 2014 10:00am – 12:00pm Plenary Session – NIH Institute Directors Grand Ballroom Chair: David Kupfer, M.D., University of Pittsburgh School of Medicine This year’s Institute Director’s session will bring together directors from various NIH institutes who all have a similar goal of searching for new approaches in the research of mental disorders. Each director will have ten minutes to discuss what activities are going on within their institute regarding this goal. Thomas Insel, NIMH Director, will begin the session discussing transformation of clinical trials. Phil Skolnick will discuss one of the more challenging issues that NIDA faces is the epidemic of (both prescription and non-prescription) opiate abuse. To put the problem in perspective, it has been estimated that there are 3 million Americans currently abusing opioids; more deaths result from opiate overdose than from firearms. He will overview NIDA’s efforts to combat both opiate abuse and overdose deaths. Kenneth Warren of NIAAA will discuss the current framework for medications development of alcohol use disorders. Josephine Briggs will discuss NCCAM’s interest in encouraging work on the neuroscience of the mind-body interface and the mechanisms by which meditative practices such as mindfulness, hypnosis, and meditative exercise forms may impact on pain processing. Christopher Austin will address NCATS’ unique role in the biomedical ecosystem and the translational science problems being prioritized by NCATS. He will also give an overview of the Center’s programs and collaborative opportunities. Finally, Richard Nakamura will discuss the Center for Scientific Review’s steps to measure and improve the performance of peer review. The session will continue with an open dialogue Q&A session with audience interaction. Thomas Insel, M.D., NIMH Phil Skolnick, Ph.D., NIDA Kenneth Warren, Ph.D., NIAAA Josephine Briggs, M.D., NCCAM Christopher Austin, M.D., NCATS Richard Nakamura, Ph.D., CSR 57 Wednesday, June 18, 2014 12:00pm – 2:00pm Poster Session II with Lunch Regency Ballroom *See Pages 81 through 92 for poster listing. 2:00pm – 3:30pm Updates Session – The Latest on Treatment of Mood, OCD-spectrum, and Binge Eating Disorders Grand Ballroom Chair: Maurizio Fava, Massachusetts General Hospital Recent advances in clinical neuroscience have led to the development of novel treatments of mood, OCDspectrum, and binge eating disorders. The purpose of this symposium is to provide an overview of the latest developments in the pharmacological treatments for these conditions. Dr. Papakostas will review new approaches to the treatment of depression, as well as to the identification of subpopulations of depressed patients more likely to benefit from a given treatment. Dr. Ketter will present an update on new therapeutic developments in the treatment of bipolar disorder, such as the approval by the FDA of asenapine, risperidone long-acting injectable (LAI), ziprasidone, aripiprazole, and lurasidone therapy for bipolar disorder. In addition, Dr. Ketter will discuss the International Society for Bipolar Disorders (ISBD) Antidepressant Use in Bipolar Disorders Task Force controversial report from 2013. Finally, Dr. Ketter will present data concerning some novel pharmacological treatments for bipolar disorder. Dr. McElroy will also provide an overview of the treatments for hoarding disorder and binge eating disorder, new discrete diagnostic entities in DSM-5. Psychological treatments are effective for both conditions, but not all patients respond and pharmacotherapy is emerging as an important treatment option. Serotonin reuptake inhibitors have been the most widely studied agents, but both conditions respond modestly at best to these compounds. Newer agents showing promise include antiepileptics and psychostimulants. Available research on the pharmacotherapy of HD and BED will be reviewed, and future directions will be discussed. 2:00pm – 2:20pm Hoarding Disorder and Binge Eating Disorder Susan McElroy, University of Cincinnati College of Medicine 58 Wednesday, June 18, 2014 2:00pm – 3:30pm (continued) Updates Session – The Latest on Treatment of Mood, OCD-spectrum, and Binge Eating Disorders Grand Ballroom 2:20pm – 2:40pm Update on Bipolar Disorder Pharmacotherapy Terence Ketter, Stanford University School of Medicine 2:40pm – 3:00pm Update on Treatment of Major Depressive Disorder George I. Papakostas, Massachusetts General Hospital, Harvard Medical School 3:00pm – 3:30pm Discussion 3:30pm – 3:45pm Break Ballroom Foyers Workshops 3:45pm – 5:45pm New Approaches to Drug Studies for Treating Social Deficits in Autism Spectrum Disorder Atlantic 1 Chair: Meg Grabb, National Institute of Mental Health Co-chair: Ann Wagner, NIMH/NIH Discussant: Alessandro Bertolino, F. Hoffmann-La Roche Ltd. 3:45pm – 3:50pm Introduction 3:50pm – 4:10pm Measuring Social Disability in Autism Spectrum Disorder Lawrence Scahill, Emory University 4:10pm – 4:30pm 4:30pm – 4:50pm Honing in on Targets for Compound Selection in ASD Trials: The NIMH FAST-ASD Network James T. McCracken, UCLA Semel Institute Incorporating Potential Functional Biomarkers in Clinical Trials in ASD Bryan H. King, Seattle Children’s Hospital and University of Washington Eye-tracking Measures of Social Disability as Outcome Measures in School-age Children with ASD Warren Jones, Marcus Autism Center 5:10pm – 5:45pm Discussion 4:50pm – 5:10pm 59 Wednesday, June 18, 2014 3:45pm – 5:45pm *Novel Mechanisms of Action for the Treatment of Depression and Anxiety: Scientific Updates Atlantic 2 Chair: Timothy Petersen, Clintara, LLC Co-chair: Jaskaran Singh, Janssen Research and Development, LLC Discussant: Randall D. Marshall, Alkermes 3:45pm – 3:50pm Introduction 3:50pm – 4:10pm A Review of Pre-clinical Data Gerard Sanacora, Yale University 4:10pm – 4:30pm Ketamine: Rationale, Empirical Evidence and New Routes of Delivery James W. Murrough, Icahn School of Medicine at Mount Sinai 4:30pm – 4:50p Treatment of Suicidality with Novel Mechanisms Sanjay Mathew, Baylor College of Medicine 4:50pm – 5:10pm The Role of Pherines for Rapid Relief of Depressive and Anxiety Symptoms Michael R. Liebowitz, Pherin Pharmaceuticals 5:10pm – 5:30pm 5:30pm – 5:45pm Novel Developments in Non-invasive Neurostimulation for the Treatment of Mood Disorders Dan V. Iosifescu, Icahn School of Medicine at Mount Sinai Discussion *of special interest to clinicians 60 Wednesday, June 18, 2014 3:45pm – 5:45pm *Psychiatry and Technology: A Partnership in Promoting Mental Health Atlantic 3 Chair: Holly A. Swartz, University of Pittsburgh School of Medicine Discussant: Ellen Frank, University of Pittsburgh School of Medicine 3:45pm – 3:50pm Introduction 3:50pm – 4:10pm MoodSwings 2.0 for Bipolar Disorder: www.moodswings.net.au Victoria E. Cosgrove, Stanford University School of Medicine 4:10pm – 4:30pm 4:30pm – 4:50pm MoodRhythm: Pilot Testing a Smartphone App for Monitoring Mood and Daily Routines Mark Matthews, Cornell University Using Smartphones to Enhance Skill Acquisition and Utilization in CBT for Child Anxiety Jennifer Silk, University of Pittsburgh Computerized Adaptive Testing (CAT) and the Future of Psychiatric Measurement Robert Gibbons, University of Chicago 5:10pm – 5:45pm Discussion 4:50pm – 5:10pm *of special interest to clinicians 61 Notes 62 Thursday, June 19, 2014 AT-A-GLANCE Thursday, June 19, 2014 7:30am – 9:00am Morning Break Ballroom Foyers 8:30am – 10:00am Panel Sessions The NIMHFunded RAPID Network Studies Leveraging the Internet and Social Media to Improve Pathways to Care and Shorten DUP in Schizophrenia Practical Trials in Psychiatry: The Need, The Opportunities Location: Diplomat 1-2 Location: Atlantic 1 Location: Atlantic 2 Location: Atlantic 3 10:00am – 10:15am Break Ballroom Foyers 10:15am – 11:45am Plenary: Regulatory Wrap-up Session Grand Ballroom 12:00pm Meeting Adjourns 63 Thursday Clinical Trials in Early Stage Alzheimer’s Disease: Current Methodological and Regulatory Considerations Notes 64 Thursday, June 19, 2014 FULL SCHEDULE Thursday, June 19, 2014 7:30am – 9:00am Morning Break Ballroom Foyers Panel Sessions 8:30am – 10:00am Clinical Trials in Early Stage Alzheimer’s Disease: Current Methodological and Regulatory Considerations Diplomat 1-2 Chair & Discussant: Kathleen Welsh-Bohmer, Joseph and Kathleen Bryan Alzheimer’s Disease Research Center 8:30am – 8:40am Introduction 8:40am – 9:00am What Are We Trying to Measure in Preclinical and Prodromal Alzheimer’s Disease? Lon S. Schneider, Keck School of Medicine of USC What are the Optimal Cognitive Outcome Measures for Trials in Preclinical Alzheimer’s Disease? Keith Wesnes, Bracket Global 9:20am – 9:40am Drug Development Cognition Challenges Marc Cantillon, Kyowa Hakko Kirin Pharma, Inc. 9:40am – 10:00am Discussion 9:00am – 9:20am 65 Thursday, June 19, 2014 8:30am – 10:00am The NIMH-Funded RAPID Network Studies Atlantic 1 Chair: Maurizio Fava, Massachusetts General Hospital Co-chair: Mi Hillefors, NIMH Discussant: Carlos A. Zarate, NIMH 8:30am – 8:40am Introduction 8:40am – 9:00am A POC of Low-field Magnetic Stimulation: Methodological Issues and Innovations in the Implementation of a Novel Device Study in MDD Maurizio Fava, Massachusetts General Hospital 9:00am – 9:20am Potential Rapid Antidepressant Augmentation with a Selective Kappa Antagonist Sitra Tauscher-Wisniewski, Lilly Research Labs A Dose-finding Study of I.V. Ketamine in Treatment-resistant Depression Gerard Sanacora, Yale University School of Medicine 9:40am – 10:00am Discussion 8:30am – 10:00am Leveraging the Internet and Social Media to Improve Pathways to Care and Shorten DUP in Schizophrenia Atlantic 2 9:20am – 9:40am Chair: John M. Kane, The Zucker Hillside Hospital Discussant: Donald C. Goff, NYU Medical School 8:30am – 8:40am Introduction 8:40am – 8:55am Duration of Untreated Psychosis and it’s Correlates in Patients with Schizophreniaspectrum Disorders: Results from a Large Metaanalysis of First Episode Studies Christoph Correll, The Zucker Hillside Hospital 8:55am – 9:10am Understanding Pathways to Care in Early-course Psychotic Disorders Michael T. Compton, Hofstra North Shore-LIJ School of Medicine at Hofstra University 66 Thursday, June 19, 2014 8:30am – 10:00am (continued) Leveraging the Internet and Social Media to Improve Pathways to Care and Shorten DUP in Schizophrenia Atlantic 2 9:10am – 9:25am Early Detection of Psychosis through Self-report Screening Rachel Loewy, University of California Reducing DUP in the Age of Social Media and the Internet John M. Kane, The Zucker Hillside Hospital 9:40am – 10:00am Discussion 8:30am – 10:00am Practical Trials in Psychiatry: The Need, The Opportunities Atlantic 3 9:25am – 9:40am Chair & Discussant: Benedetto Vitiello, NIMH Co-chair: Nina R. Schooler, SUNY Downstate Medical Center 8:30am – 8:40am Introduction 8:40am – 8:55am Practical, Pragmatic, and Possible Psychopharmacology Clinical Trials: Lessons Learned from the AHRQ Bipolar CHOICE study Andrew Nierenberg, Massachusetts General Hospital 8:55am – 9:10am 9:10am – 9:25am Expanding the Place of Practice-based Clinical Trials on the Explanatory-pragmatic Spectrum Greg Simon, Group Health Research Institute A Regulatory Perspective on Practical Clinical Trials Thomas Laughren, MGH CTNI 9:25am – 9:40am PCORI and Practical Trials in Mental Health Grayson Norquist, The University of Mississippi Medical Center 9:40am – 10:00am Discussion 67 Thursday, June 19, 2014 10:00am – 10:15am Break Ballroom Foyers 10:15am – 11:45am Plenary: Regulatory Wrap-up Session Grand Ballroom Chair: Ni A. Khin, M.D., U.S. Food and Drug Administration Phillip Kronstein, M.D., Food and Drug Administration Celia Winchell, M.D., Food and Drug Administration Silvana Borges, M.D., Food and Drug Administration 12:00pm Meeting Adjourns 68 Posters Tuesday, June 17, 2014 Poster Session 1 A Double-blind, Randomized, Placebo-controlled, Parallel Group, Dose Frequency Study of Intravenous Ketamine in Patients with Treatment-Resistant Depression Jaskaran Singh, Janssen Research and Development, LLC P-2 A Pilot Study of a Novel Monoamine Triple Reuptake Inhibitor EB-1020 SR in the Treatment of ADHD in Adults Timothy Wilens, Massachusetts General Hospital Andrew J. Cutler, Ann Childress, Randall D. Marshall, Mark Bradshaw, Frank Bymaster, Anthony McKinney, Stephen W. Hurt, Catherine O’Brien, Timothy Hsu P-3 AZD8529, a Positive Allosteric Modulator of the mGiuR2 Receptor for the Treatment of Schizophrenia Alan Cross, AstraZeneca Neuroscience Innovative Medicines Unit P-4 Efficacy and Safety of a Novel mGlu2 Receptor Positive Allosteric Modulator as an Adjunctive Treatment to an SSRl/SNRI in the Treatment of Anxious Depression Justine Kent, Janssen Ella Daly, Ceusters Marc, Iva Kezic, Rosanne Lane, Lim Pilar, De Smedt Heidi, Mazzucco Christine, Peter DeBoer, Luc Van Nueten, Wayne Drevets P-5 Lupron in Combination with an Acetylcholinesterase Inhibitor Halts Cognitive Decline in Women with Alzheimer ‘s Disease over a 48 Week Period Richard Bowen, OTB Research Craig Atwood P-6 Results of a Phase 28 Clinical Trial of TC-5619, a Selective Alpha 7 Neuronal Nicotinic Receptor (NNR) Agonist in the Adjunctive Treatment of Negative Symptoms and Cognitive Dysfunction in Schizophrenia David Hosford, Targacept, Inc. Chris Dvergsten, Jessica Beaver, Anthony Segreti, Steven Toler, Gaston Farr, Melissa Joseph, John Jett, Patrick Lippiello, Merouane Bencherif P-7 A Rapidly Acting Intranasal Treatment for the Symptoms of GAD Michael Liebowitz, Pherin Pharmaceuticals Louis Monti, Rita Hanover, Bernard Grosser P-8 Metadoxine Extended Release (MDX): A Novel Drug Candidate for the Treatment of ADHD & Other Cognitive Disorders Jonathan Rubin, Alcobra Pharma Yaron Daniely, Lenard Adler *P-# References a Pharmaceutical Pipeline Presentation. 69 Posters P-1 Posters 1 Memantine in the Treatment of Executive Function Deficits in Adults with ADHD: A Pilot Randomized Double Blind Controlled Clinical Trial Joseph Biederman, Massachusetts General Hospital Ronna Fried, Laura M. Tarko, Craig Surman, Stephen V. Faraone, Thomas Spencer 2 Effects of Lisdexamfetamine Dimesylate on Brain Reward Circuitry in Adults with ADHD Stephanie Duhoux, Icahn School of Medicine at Mount Sinai Kurt Schulz, Beth Krone, Anne-Claude V. Bédard, Juan D. Pedraza, Lenard Adler, Stuart F. White, James Blair, Jeffrey Newcorn 3 The Methylphenidate-atomoxetine Crossover Study in ADHD Youth: Measuring and Comparing Response Mark Stein, The University of Washington Tom Hildebrandt, Jeffrey Newcorn 4 The Role of Aldosterone and Cortisol in Alcohol Use Disorders in a Baclofen Treatment Study Elie G. Aoun, Brown University, Butler Hospital Carolina Haass-Koffler, Robert Swift, Giovanni Addolorato, George Kenna, Lorenzo Leggio 5 Addiction Severity Index Family Composite Scale More Reliable Than Combined Family/Social Composite Wayne H. Denton, Florida State University Jacob B. Priest, Sarah B. Woods 6 Periodic Placebo Effect in an Addiction Therapy Trial Bernard L. Silverman, Alkermes, Inc. Jacqueline Zummo, Asli Memisoglu, David Gastfriend, Walter Ling, Evgeny Krupitsky 7 Adrenergic Receptor Modulation for the Treatment and Prevention of Post-traumatic Stress Disorder and Co-morbid Disorders Chaya G. Bhuvaneswaran, University of Massachusetts-Worcester 8 An Epigenome-wide Assessment of Atypical Antipsychotic Side Effects in Bipolar Disorder Kyle J. Burghardt, University of Michigan College of Pharmacy Vicki L. Ellingrod = New Investigator 70 Posters 9 Biomarkers of Cardiometabolic Risk in Antipsychotic Treated Youth Ginger E. Nicol, Washington University School of Medicine Michael D. Yingling, Julia A. Schweiger, John Newcomer 10 Plasma Polyunsaturated Fatty Acid Markers Differ in Symptomatic Bipolar Disorder Erika Saunders, Penn State College of Medicine, Penn State University Kaizong Ma, Eric Schaefer, Alan Gelenberg, Stanley Rapoport 11 Reliable Change Index and Clinical Significance in Clinical Trials Using the Positive and Negative Syndrome Scale (PANSS) Mark Opler, ProPhase LLC, New York University School of Medicine Anzalee Khan, Linda Gao, Brian Rothman, Luka Lucic 12 Prenatal Stress Evokes Long-term Changes in Brain Glucose Metabolism Boguslawa Budziszewska, Institute of Pharmacology, Polish Academy of Sciences Anna Kurek, Jan Detka, Agnieszka Basta-Kaim, Monika Leśkiewicz, Marta Kubera 13 Efficacy and Safety of Treatment with Lurasidone Adjunctive with Lithium or Valproate in Bipolar I Depression: Results of Two 6-week Studies Joseph R. Calabrese, Case Western Reserve School of Medicine Trisha Suppes, Kaushik Sarma, Robert Silva, Hans Kroger, Josephine Cucchiaro, Andrei Pikalov, Antony Loebel 14 MoodSwings 2.0 (www.moodswings.net.au): An Online Intervention for Bipolar Disorder Victoria E. Cosgrove, Stanford University School of Medicine Trisha Suppes, Sue Lauder, Emma Gliddon, Karishma Raju, Seetal Dodd, E. Grace Fischer, Michael Berk 15 Clinically Relevant Change Using CGI-BP in Patients with Acute Depressive Episodes of Bipolar I or II Disorder in Quetiapine XR Study Catherine Datto, AstraZeneca Jason Wright, Scott LaPorte, Michelle Shay 71 Posters 16 Number Needed to Treat for Discontinuation Due to Adverse Events, Somnolence, ≥ 7% Weight Gain, Extrapyramidal Side Effects, Response, and Remission of Atypical Antipsychotics in Acute Bipolar Depression Keming Gao, Case Western Reserve University 17 Lurasidone in Bipolar I Depression: A 24 Week, Open-label Extension Study Terence Ketter, Stanford University School of Medicine Kaushik Sarma, Robert Silva, Hans Kroger, Josephine Cucchiaro, Antony Loebel 18 Sleep Patterns across the Bipolar Spectrum: Similarities and Differences between Mood States and Across Diagnostic Subtypes Jessica C. Levenson, University of Pittsburgh Holly A. Swartz, Ellen Frank, David J. Kupfer 19 Global Improvement in Bipolar Mania Patients Treated with Cariprazine Robert E. Litman, CBH Health, LLC Kaifeng Lu, Krisztián Nagy, István Laszlovszky, Suresh Durgam 20 Psychopharmacology Algorithm for Acute Mania David Osser, Harvard Medical School Othman Mohammad 21 The Psychopharmacology Algorithm Project at the Harvard South Shore Program: 2014 Update on Bipolar Depression Dana Wang, Harvard Medical School, VA Boston Healthcare System Arash Ansari, David Osser 22 Correlation between Different Levels of Placebo Response Rate and Clinical Trial Outcome in Bipolar Depression George I. Papakostas, Massachusetts General Hospital, Harvard Medical School Nadia Iovieno, Rosemary Walker 23 Resilience in High-risk Infants and Toddlers of Mothers with Bipolar Disorder: A Longitudinal Investigation Diana I. Simeonova, Emory University School of Medicine Theresa Nguyen, Kerry Ressler, W. Edward Craighead = New Investigator 72 Posters 24 A Dimensional Assessment of Anxiety and Tic Severity in Tourette’s Disorder Barbara Coffey, Icahn School of Medicine at Mount Sinai, Nathan S. Kline Institute for Psychiatric Research Vilma Gabbay 25 Computerized Adaptive Diagnosis and Testing in Psychiatric Outpatients Seeking Care at a Large, Free-standing Psychiatric Hospital Eric D. Achtyes, Michigan State University College of Human Medicine Scott Halstead, LeAnn D. Smart, Robert D. Gibbons 26 A Dimensional Rating System for Psychiatric Disorders in Psychiatric Outpatients Mark Zimmerman, Rhode Island Hospital 27 A Clinically Useful Self-report Measure of the DSM-5 Anxious Specifier of Major Depressive Disorder Mark Zimmerman, Rhode Island Hospital 28 Reduction of Placebo Response in Depression Trials via Independent Remote (SAFER) Patient Interviews Martina J. Flynn, Massachusetts General Hospital Clinical Trials Network and Institute Marlene Freeman, Maurizio Fava, David Mischoulon, James Pooley, Daniel Burch, Heather Bryson 29 A Comparison of Cross-cultural Regional Norms for the MATRICS Consensus Cognitive Battery (MCCB) Richard S. E. Keefe, NeuroCog Trials, Duke University Ioan Stroescu, Vicki G. Davis, Alexandra S. Atkins 30 Magnitude of Change with Antidepressants and Placebo in Antidepressant Clinical Trials Using Structured, Taped and Appraised Rater Interviews Compared to Traditional Semistructured Interviews Arif Khan, Northwest Clinical Research Center James Faucett, Walter Brown 31 The Impact of Implementing a National Research Subject Database to Prevent Dual Enrollment in Early and Late Phase Central Nervous System Trials Kerri Weingard, Accumed Research Associates & Verified Clinical Trials Mitchell Efros = New Investigator 73 Posters 32 Early Life Stress as a Risk Factor for Substance Use Disorders: Clinical and Neurobiological Substrates Sajoy Purathumuriyil Varghese, Rosalind Franklin University of Medicine and Science 33 Panic Disorder: Theoretical Overlap with Narcolepsy Thomas W. Uhde, MUSC Bernadette M. Cortese, Priyattam Shiromani, Orlena Merritt-Davis, Yury Yaroslavsky, Ravi Singareddy, Kimberly R. Leslie, Martha Strachan, Jennifer Runion, David Bachman, Richard K. Bogan 34 A Five Year Observational Study of Patients with Treatment Resistant Depression Treated with VNS Therapy® or Treatment as Usual: Comparative Response/Remission Rates, Duration of Response, and Quality of Life Scott T. Aaronson, Sheppard Pratt Health System Mark T. Bunker, Peter Sears, Francis Ruvuna 35 Prenatal Stress Influences the Proper Functioning of the Primary Microglial Cells and Leads to Behavioral Changes in Adult Offspring - A Link to Depression Agnieszka Basta-Kaim, Institute of Pharmacology, Polish Academy of Sciences Joanna Slusarczyk, Bogusława Budziszewska, Monika Leśkiewicz, Marta Kubera 36 The Efficacy of Vilazodone in Achieving Remission in Patients with Major Depressive Disorder: Post Hoc Analyses of a Phase IV Trial Leslie Citrome, New York Medical College Carl Gommoll, Xiongwen Tang, Rene Nunez, Maju Mathews 37 Clinical Relevance of Levomilnacipran ER Treatment in Patients with Major Depressive Disorder: Improvements in Functional Impairment Categories Andrew J. Cutler, Florida Clinical Research Center, LLC Carl Gommoll, Changzheng Chen, William M. Greenberg, Adam Ruth 38 Levomilnacipran Inhibits both Norepinephrine and Serotonin Reuptake across the Clinical Dose Range Tobie Escher, Forest Research Institute Joann O’Connor, Laishun Chen, Carl Gommoll, Stephen Zukin 74 Posters 39 Increased ACC and Striatal Total Choline Levels in Adolescent Depression Vilma Gabbay, Icahn School of Medicine at Mount Sinai; Nathan S. Kline Institute for Psychiatric Research Ana I. Vallejo, Amy R. Johnson 40 Cognitive Domains Impacted by Vortioxetine Treatment of Patients with Major Depressive Disorder (MDD) John E. Harrison, Metis Cognition Ltd. Søren N. Lophaven, Christina Kurre Olsen 41 A Head-to-head, Randomized, Comparison Study of Vortioxetine vs. Escitalopram in Patients Well Treated for MDD and Experiencing Treatment-emergent Sexual Dysfunction Paula L. Jacobsen, Takeda Development Center Americas Atul R. Mahableshwarkar, Yinzhong Chen, Lambros Chrones, Anita Clayton 42 The Role of Pro-inlammatory Cytokines in Modulation of Activity of Serotoninergic System in Women with Postpartum Depressive Symptoms and in Animal Models of Depression Marta Kubera, Institute of Pharmacology, Polish Academy of Sciences Katarzyna Curzytek, Weronika Duda, Joanna Slusarczyk, Monika Leskiewicz, Krystyna Golembiowska, Magdalena Regulska, Agnieszka Basta-Kaim, Bogusława Budziszewska, Wladyslaw Lason, Michael Maes 43 Anti-anhedonic Effect of Ketamine and its Neural Correlates in Major Depressive Disorder Níall Lally, National Institutes of Health Allison C. Nugent, David Luckenbaugh, Carlos Zarate 44 ALKS 5461, a Novel Opioid Modulator as Adjunctive Treatment for Depression: Addressing Abuse Potential, Safety and Efficacy Randall D. Marshall, Alkermes, Inc. Ryan Turncliff, J. Alexander Bodkin, Lauren E. DiPetrillo, Richard Leigh-Pemberton, Michael E. Thase, Madhukar Trivedi, Asli Memisoglu, Maurizio Fava 45 Effects of Vilazodone on Sexual Dysfunction in Major Depressive Disorder: A Randomized, Double-blind Trial with Placebo and Active Controls Maju Mathews, Forest Research Institute Carl Gommoll, Dalei Chen, Rene Nunez 75 Posters 46 A 8-week Randomized, Double-blind Trial Comparing Efficacy, Safety and Tolerability of Three Vilazodone Dose Initiation Strategies Following Switch from SSRIs or SNRIs in Major Depressive Disorder Robert Millet, Duke University Shilpa Rele, Sungman Kim, Jong-Woo Paik, Seonghwan Kim, Varun Kasula, Prakash Masand, Ashwin Patkar 47 Adjunctive Lanicemine (AZD6765) in Patients with Major Depressive Disorder and a History of Inadequate Response to Antidepressants: Post-hoc Analyses of a Randomized, Placebo Controlled Study (PURSUIT) Sanjeev Pathak, AstraZeneca Hong-Lin Su, Joel Posener, Khanh Bui, Michael Quirk, Tim Piser, Sanjay Mathew, Gerard Sanacora 48 The Rosenberg Hassman Mood Scale - An Update on the Development of This Depression Rating Scale with Feedback from 50 Patients Leon I. Rosenberg, Center for Emotional Fitness Keith Wesnes, Sanju George, Howard Hassman, Mary Gelovich 49 Efficacy of Vortioxetine vs. Placebo in Adults with Major Depressive Disorder (MDD): Meta-analyses of MADRS Single Items from 9 Short-term Studies Michael E. Thase, Perelman School of Medicine of the University of Pennyslvania Atul R. Mahableshwarkar, Henrik Loft, Marianne Dragheim 50 mTOR Signaling Correlates with Treatment Response to Ketamine in a Preclinical Model of Treatment Resistant Depression Susannah J. Tye, Mayo Clinic Adam Walker, Blair Price, Chunling Hu, Shari Sutor, Mark A. Frye 51 A Novel Trial Design to Assess Rapid and Sustained Antidepressant Effects of an Oral NR2B Specific NMDA Receptor Antagonist, CERC-301 James Vornov, Cerecor, Inc. Maurizio Fava, Michael Detke, Chao Wang, Larry Ereshefsky, Richard C. Shelton, Michael E. Thase, Madhukar Trivedi 76 Posters 52 An International Study of the GRID-HAMD: Has It Fulfilled Its Promise? Janet Williams, MedAvante, Inc. Matej Ondrus, Melanie Rishton, Jennie K. Persson, Marlene Popescu, Risto Valjakka 53 Validation of the Yale-Brown Obsessive Compulsive Scale Modified for Binge Eating to Support Use in Clinical Trials as a Measure of Treatment Benefit Linda Deal, Shire Development LLC Robert Wirth, Barry Herman, Maria Gasior, Susan McElroy 54 Acquired Binge-eating Behaviour Produces Alterations in Dopaminergic Neurochemistry in the Brains of Rats Peter Hutson, Shire Jane Gosden, Mike Prow, David J. Heal, Sharon Cheetham 55 Definitive Verification and Monitoring of Oral Medication Adherence Using Breath Analysis Donn M. Dennis, Xhale SMART, Inc. Eileen Loskie 56 Predictors of Pharmacological Treatment Response in Grooming Disorders Brian L. Odlaug, University of Copenhagen Jon E. Grant, Eric Leppink, Katherine L. Derbyshire 57 Web-based Curriculums for Teaching Psychopharmacology: Revision of the Resident and the Medical Student Curriculums Ira Glick, Stanford University School of Medicine 58 The Effects of Social Support on Suicidality in an Adult Inpatient Psychiatric Population as Assessed by the C-SSRS and S-STS Ahmad Hameed, Penn State College of Medicine Amanda M. White, Michael Mitchell, Eric A. Youngstrom, Roger E. Meyer, Alan J. Gelenberg 59 Deconstructing Drug Company Promotion: Pursuing Truth: Slides for a 1 Hour Lecture in a Psychopharmacology Course Jeffrey Mattes, Psychopharmacology Research Association of Princeton 77 Posters 60 Metabotropic Glutamate Receptor 7 (GRM7) Pharmacogenetics in First Episode Psychosis James M. Stevenson, University of Illinois College of Pharmacy James L. Reilly, Margret S. H. Harris, Konasale M. Prasad, Judith A. Badner, Vishwajit Nimgaonkar, Matcheri S. Keshavan, John A. Sweeney, Jeffrey R. Bishop 61 Medical Informatics in Psychiatric Practice: Current Status and Unmet Needs Farifteh Duffy, American Psychiatric Association/American Psychiatric Foundation Laura Fochtmann, Robert M. Plovnick, Diana E. Clarke, Eve K. Mościcki, William Narrow 62 All-cause Discontinuation and Safety of Aripiprazole Oncemonthly for the Treatment of Schizophrenia: A Pooled Analysis of Two Double-blind, Randomized, Controlled Trials Ross A. Baker, Otsuka Pharmaceutical Development & Commercialization, Inc. W. Wolfgang Fleischhacker, Raymond Sanchez, Lan-Feng Tsai, Timothy Peters-Strickland, Anna Eramo, John Kane 63 Subject Recruitment Strategies: A New Cross-functional Team Approach Kim Cheshire-Kinney, Janssen Research & Development, LLC Stephen Rodriguez, James O’Neill, Lucy Mahalchick, Allen Wu 64 Accelerated Aging in Severe Mental Illness Using Levels of Advanced Glycated Endproducts as Indicator. Research Findings and Clinical Consequences Dan Cohen, Mental Health Organization North-Holland North Annet Nugter, Andries Smit 65 A Double-blind, Placebo-controlled, Randomized Withdrawal Study of Lurasidone for the Maintenance of Efficacy in Patients with Schizophrenia Josephine Cucchiaro, Sunovion Pharmaceuticals, Inc. Rajiv Tandon, Antony Loebel, Debra Phillips, David Hernandez, Yongcai Mao, Andrei Pikalov 66 Categorical Improvements in Disease Severity in Schizophrenia Patients Treated with Cariprazine Suresh Durgam, Forest Research Institute Stephen Zukin, Kaifeng Lu, Marc Debelle, István Laszlovszky, Stephen Volk 78 Posters 67 Efficacy and Safety of Aripiprazole Once-monthly in Obese and Non-obese Patients with Schizophrenia: A Post Hoc Analysis Anna Eramo, H. Lundbeck A/S Marc De Hert, Wally Landsberg, Lan-Feng Tsai, Ross A. Baker 68 The Effects of Caloric Vestibular Stimulation on Illness Awareness in Schizophrenia: A Pilot, Proof of Concept Study Philip Gerretsen, Centre for Addiction and Mental Health David D. Pothier 69 Comparative Outcomes after Switching from Risperidone Longacting Injectable to Paliperidone Long-acting Injectable or Oral Antipsychotics David Hough, Janssen Research & Development Erica Voss, Larry Alphs, Patrick Ryan, Paul Stang 70 Exploring Neuropathological Deficits and New Drug Targets for Major Psychiatric Disorders Using the Stanley Neuropathology Consortium Datasets and RNA-Seq Data Sanghyeon Kim, Stanley Medical Research Institute 71 Clozapine May Exert Its Superior Efficacy on Schizophrenia through Its Serotonin 5HT2C Receptor Inverse-agonism Sanghyeon Kim, Stanley Medical Research Institute 72 An Open-label Extension Study of Lurasidone Safety and Efficacy in Patients with Schizophrenia Previously Randomized to Lurasidone or Risperidone Andrei Pikalov, Sunovion Pharmaceuticals, Inc. Gregory Mattingly, Michael Tocco, Debra Phillips, Jane Xu, Antony Loebel 73 Correlates or Social Cognition and Neurocognition to Functional Outcomes Brian Rothman, ProPhase, LLC Jean-Pierre Lindenmayer, Anzalee Khan, Mark Opler, Linda Gao 74 A Randomized Blind Parallel Intramuscular Haloperidol-controlled Multicenter Clinical Trial to Evaluate the Efficacy and Safety of Intramuscular Levosulpiride in the Treatment of Chinese Patients with Agitation of Schizophrenia Yifeng Shen, Shanghai Mental Health Center Huafang Li = New Investigator 79 Posters 75 Varenicline Effects on Smoking, Cognition, and Psychiatric Symptoms in Schizophrenia: Results of a Double-blind Placebo Controlled Study Robert C. Smith, NYU School of Medicine and Nathan Kline Institute for Psychiatric Research 76 Paliperidone Research in Demonstrating Effectiveness (PRIDE): Managing Schizophrenia Patients with a History of Incarceration H. Lynn Starr, Janssen Scientific Affairs, LLC Larry Alphs, Lian Mao, Stephen Rodriguez 77 A Randomized, Placebo-controlled Repeat-dose Thorough QT Study of Inhaled Loxapine in Healthy Volunteers Paul P. Yeung, Teva Pharmaceuticals James V. Cassella, Daniel A. Spyker 78 Lamotrigine: Are We Dosing it Optimally in Pregnant Women with Bipolar Disorder? Crystal T. Clark, Northwestern University Feinberg School of Medicine 79 Sexual Symptoms Associated with Leuprolide Acetate Therapy in Infertility Patients Treated for Endometriosis Julia “Jill” K. Warnock, University of Oklahoma-HSC- Tulsa J. Clark Bundren 80 Posters Wednesday, June 18, 2014 Poster Session 2 1 A Novel Computer-prompted Tandem Rating Assessment for Adult ADHD Clinical Trials Joan Busner, Penn State College of Medicine and Bracket Andrew J. Cutler, Gary S. Sachs, Dan DeBonis, Timothy Hsu, Catherine O’Brien, Timothy Wilens 2 Emotional Dysregulation as an Adult ADHD Subtype Frederick W. Reimherr, Psychiatric & Behavioral Solutions, University of Utah Tammy A. Steans, Kathleen Reimherr, Phillip D. Gale, Thomas Gift, Paul H. Wender, Barrie K. Marchant 3 Emotion Recognition Deficits in Treated and Untreated Adult ADHD Patients Anneka Tomlinson, University of Manchester Robert Baskind, Joe Johnson, Kay Marshall, Joanna C. Neill 4 Attenuation of Ethanol Withdrawal by Ceftriaxone-induced Upregulation of Glutamate Transporter EAAT2 Ulas M. Camsari, Mayo Clinic Osama Abulseoud 5 A Double-blind, Placebo-controlled Trial Assessing the Efficacy of Varenicline Tartrate for Alcohol Dependence Raye Z. Litten, NIAAA Joanne B. Fertig, Daniel E. Falk, Megan L. Ryan 6 Progesterone Treatment for Postpartum Cocaine Users Kimberly Yonkers, Yale School of Medicine Mehmet Sofuoglu, Kathleen Carroll 7 Desvenlafaxine ER vs. Placebo in Social Anxiety Disorder Michael R. Liebowitz, Pherin Pharmaceuticals Ester Salman, Ann E. Johnson, Rita Hanover 8 The Predictive Value of Gene Variants Used to Guide Antidepressant Selection Kevin M. Furmaga, Pine Rest CMHS and Michigan State University College of Human Medicine (Psychiatry) Andrew C. Rose, LeAnn D. Smart, Alan T. Davis, Eric D. Achtyes 81 Posters 9 Determining Pharmacological Selectivity of the Kappa Opioid Receptor Antagonist LY2456302 Using Translational Rat to Human Pupillometry Studies Linda Rorick-Kehn, Lilly Research Laboratories, Eli Lilly and Co 10 A Naturalistic Study of the Clinical Utility of Pharmacogenetic Testing in Psychiatric Patients Rachel Scott, Genomind Herbert Harris, Kathryn Gardner, Jay Lombard, Francis X. Brennan 11 How Cardinal are Cardinal Symptoms in Pediatric Bipolar Disorder? A Familial Risk Analysis Joseph Biederman, Massachusetts General Hospital Stephen V. Faraone, Laura M. Tarko, Mariely Hernandez, Janet Wozniak 12 High EPA Omega-3 Fatty Acids and Inositol as Monotherapy and in Combination in the Treatment of Pediatric Bipolar Disorder: A Pilot Double-blind Randomized Clinical Trial Joseph Biederman, Massachusetts General Hospital Stephen V. Faraone, Laura M. Tarko, Mariely Hernandez, Janet Wozniak 13 Identifying Patients Meeting the DSM-5 Criteria for Bipolar Disorder Episodes with Mixed Features in Bipolar Disorder Studies with Quetiapine XR Catherine Datto, AstraZeneca Jason Wright, Scott LaPorte, Michelle Shay 14 Lurasidone Monotherapy for Bipolar Depression: Influence of Baseline Thyroid Function on Treatment Response Joseph F. Goldberg, Icahn School of Medicine at Mount Sinai Andrei Pikalov, Kei Watabe, Antony Loebel 15 Screening and Validation of Novel Kinase Signaling Pathways for Neuronal Excitability Wei-Chun Hsu, University of Texas Medical Branch at Galveston Miroslav Nenov, Alexander Shavkunov, Neli Panova-Elektronova, Fernanda Laezza 16 Mediators of Effects of Lurasidone on Functioning and Quality of Life: Results from a Randomized, Double-blind, Placebocontrolled Trial in Patients with Bipolar I Depression Terence Ketter, Stanford University School of Medicine Cynthia Siu, Krithika Rajagopalan, Andrei Pikalov, Antony Loebel 82 Posters 17 Predictors of Improvement in Quality of Life Associated with Lurasidone Treatment of Bipolar I Depression: Results from a 6-month Continuation Study Terence Ketter, Stanford University School of Medicine Cynthia Siu, Mariam Hassan, Krithika Rajagopalan, Andrei Pikalov, Antony Loebel 18 Baseline Patient Characteristics of Bipolar II Compared to Bipolar I Disorder in Trials of Acute Bipolar Depression Jamie Mullen, AstraZeneca Catherine Datto, Louisa Feeley, Scott LaPorte 19 Neural Correlates of Social Stress in Youth with Bipolar Disorder Donna Roybal, University of Texas Health Sciences Center at San Antonio Amy Garrett, Victoria E. Cosgrove, Spencer Boucher, Jennifer Pearlstein, Paige Staudenmaier, Jade Garneau-Fournier, Amy Parkinson, Kiki Chang 20 The Young Mania Rating Scale in Bipolar Disorder: Evaluation of Sleep and Rater Training Jan Sedway, inVentiv Health Clinical Cristina Maneru, Sandor Palfi 21 Factors Influencing the Diagnosis and Treatment of Bipolar Depression: A Healthcare Professional Perspective Purvi K. Smith, Health and Wellness Partners Andrei Pikalov, Gary S. Sachs, Jani Hegarty 22 Sequence Analysis of Drug Target Genes with Suicide Severity in Bipolar Disorder Clement Zai, Centre for Addiction and Mental Health, University of Toronto Vanessa Goncalves, Vincenzo De Luca, Arun K. Tiwari, John B. Vincent, James Kennedy 23 Immunological Stress Responsivity as a Potential Risk Factor in Pediatric Mood Disorders Victoria E. Cosgrove, Stanford University School of Medicine Staudenmaier Paige, Jennifer Pearlstein, Sherrie Li, Kiki Chang = New Investigator 83 Posters 24 Inter-rater Reliability of the Scales for Outcomes of Parkinson’s Disease – Cognition (SCOPA-COG) in MODERATO: A Randomized, Placebo-controlled Trial to Assess the Effect of Rasagiline on Mild Cognitive Impairment in PD Patients Kari Nations, INC Research Robin C. Hilsabeck, Russell Tanenbaum, ElizaBeth Grubb, Azhar Choudhry 25 Risk-based Monitoring for Aberrant Rating Patterns and Patient Selection Anomalies in Global Schizophrenia Trials David Daniel, Bracket Global, LLC Alan Kott 26 Feasibility, Integrity and Efficiency of the Sequential Parallel Comparison Clinical Trial Design Marc de Somer, Alkermes, Inc. Yangchun Du, Asli Memisoglu, Randall D. Marshall, Richard LeighPemberton, Bernard L. Silverman, Elliot Ehrich, Maurizio Fava 27 Blinded Dual Ratings Confirm Primary Site-based Ratings in an MDD Trial Richard Leigh-Pemberton, Alkermes, Inc. Asli Memisoglu, Steven D. Targum, J Cara Pendergrass, Philip Rauh, Randall D. Marshall, Bernard L. Silverman, Marc de Somer, Elliot Ehrich 28 Complexity in Protocol Design: Does It Lead to Better Clinical Trial Outcomes? Robert Molpus, CNS Healthcare Patricia Brown, Rebecca Hummel, Mark Joyce, Linda Harper, Terri Wood, Angela Menosky, John D. Ehrhardt, Ruth Hummel 29 Impact of BPRS Interview Length on Ratings Precision during a Schizophrenia Trial Steven D. Targum, Clintara, LLC J. Cara Pendergrass, Laura Zumpano, Philip Rauh, Nicholas DeMartinis 30 Attenuation of Impulsivity in Bipolar Alcoholics Who Reduce Heavy Drinking: Prospective Evidence from a Randomized Placebo-controlled Trial Bryan Tolliver, Medical University of South Carolina James J. Prisciandaro, Delisa Brown, Helena Brenner 84 Posters 31 Efficacy of Quetiapine-XR Monotherapy or Adjunctive Therapy to Antidepressant in Acute Major Depressive Disorder with Generalized Anxiety Disorder: A Randomize, Placebo-controlled Pilot Study Keming Gao, Case Western Reserve University 32 Does Algorithm-based Depression Care Mitigate Cognitive Decline in Older Adult Outpatients? Aaron M. Koenig, Western Psychiatric Institute & Clinic Meryl A. Butters, Charles F. Reynolds 33 Pro-cognitive Effects of Ketamine and Underlying Neurocircuitry in Subjects with MDD as Assessed by fMRI and Neuropsychological Testing Lynnette A. Averill, Yale School of Medicine, National Center for PTSD 34 Confirming MDDScore as an Aid in the Diagnosis of Major Depressive Disorder John A. Bilello, Ridge Diagnostics, Inc. Linda M. Thurmond, Katie Smith, Robert A. Rubin, Suzin M. Wright, Floyd Taub, Michael E. Henry, Richard C. Shelton, George I. Papakostas 35 The Effect of Vortioxetine on Sexual Dysfunction during the Treatment of Adults with Major Depressive Disorder (MDD) or Generalized Anxiety Disorder (GAD) Anita H. Clayton, University of Virginia Paula L. Jacobsen, Atul R. Mahableshwarkar, William Palo, Yinzhong Chen, Marianne Dragheim 36 The Effects of Buprenorphine and Samidorphan, Alone and in Combination, on Monoamine Release within the Nucleus Accumbens Shell and Medial Prefrontal Cortex of Male Wistar Rats Daniel Deaver, Alkermes, Inc. Jacobi I. Cunningham, Mark S. Todtenkopf, Reginald L. Dean, David Eyerman 37 The Neurocircuitry of Increased Inflammation in Depression: Preliminary Findings Jennifer C. Felger, Emory University School of Medicine Zhihao Li, Ebrahim Haroon, Bobbi Woolwine, Andrew H. Miller = New Investigator 85 Posters 38 Anhedonia and Irritability as Correlates of Adverse Clinical Features in Adolescent Major Depression Vilma Gabbay, Icahn School of Medicine at Mount Sinai, Nathan S. Kline Institute for Psychiatric Research Amy R. Johnson, Ana I. Vallejo, Amira Hanna 39 A Dual-probe Microdialysis Investigation of the Interaction Between Lisdexamfetamine Dimesylate (LDX) and S-citalopram on CNS Monoamines – Evidence for Synergistic Augmentation of Serotonin and Dopamine Efflux Peter Hutson, Shire David Heal, Helen L. Rowley, Rajiv S. Kulkarni 40 Determination of the Monoaminergic Interactions between Lisdexamfetamine Dimesylate (LDX) and Duloxetine Reveals a Synergistic Augmentation of Dopamine Efflux in the Nucleus Accumbens and Striatum Peter Hutson, Shire Helen L. Rowley, Rajiv Kulkarni, David J. Heal 41 Safety and Tolerability of Vortioxetine 15 and 20 mg in Subjects with Major Depressive Disorder (MDD): A Phase 3, Long-term, Open-label Extension Study Paula L. Jacobsen, Takeda Development Center Americas, Inc. Linda Harper, Michael Serenko, Serena Chan, Atul R. Mahableshwarkar 42 Efficacy and Safety of Vilazodone 20 Mg and 40 Mg in Major Depressive Disorder: A Randomized, Double-blind, Placebo- and Active-controlled Trial Arif Khan, Northwest Clinical Research Center Carl Gommoll, Maju Mathews, Dalei Chen, Rene Nunez 43 Optimizing the Response to TMS in Major Depression through Intensive Concomitant Medication Management Kimberly M. Lavigne, Louisiana State University James G. Barbee, Tonya C. Hansel, Joshua F. Jansen, Jose CalderonAbbo 44 Edivoxetine as Adjunctive Treatment for Patients with Major Depressive Disorder Who Are Partial Responders to Selective Serotonin Reuptake Inhibitor Treatment: 3 Randomized, Placebocontrolled, Double-blind Studies James M. Martinez, Eli Lilly and Company Susan G. Ball, Lauren B. Marangell, Margaret B. Ferguson, Beth A. Pangallo, Mary Anne Dellva, Celine Goldberger 86 Posters 45 Effects of Vilazodone on Sexual Dysfunction in Major Depressive Disorder: A Randomized, Double-blind Trial with Placebo and Active Controls Maju Mathews, Forest Research Institute Carl Gommoll, Dalei Chen, Rene Nunez 46 Do the Dissociative Side Effects of Ketamine Mediate Its Antidepressant Effects? Mark J. Niciu, National Institutes of Health, National Institute of Mental Health David Luckenbaugh, Dawn F. Ionescu, Neal Nolan, Erica M. Richards, Nancy Brutsche, Sara Guevara, Carlos Zarate 47 In MDD Patients Switched After an Inadequate Response, the Efficacy and Tolerability of Vortioxetine versus Agomelatine is Independent of Previous Antidepressant Treatment George I. Papakostas, Massachusetts General Hospital, Harvard Medical School Rebecca Z. Nielsen, Marianne Dragheim 48 Adjunctive Lanicemine (AZD6765) in Patients with Major Depressive Disorder and a History of Inadequate Response to Antidepressants: Primary Results from a Randomized, Placebocontrolled Study (PURSUIT) Gerard Sanacora, Yale University School of Medicine Michael Johnson, Arif Khan, Sarah D. Atkinson, Robert Riesenberg, Juan Schronen, Michael A. Burke, John Zajecka, Hong-Lin Su, Sanjay Mathew, Sanjeev Pathak 49 Impact of a Culturally-focused Psychiatric Consultation on Depressive Symptoms among Spanish- and English-speaking Latinos in Primary Care Nhi-Ha Trinh, Depression Clinical and Research Program, Massachusetts General Hospital Ilana Huz, Lara Traeger, Trina Chang, Maurizio Fava, Albert Yeung, Stephen Gilman 50 Association between Physicians’ Expectations and Clinical Response: Re-analysis of Data from the Hypericum Depression Trial Study Group Sagar A. Vijapura, Harvard Medical School Justin Chen, George I. Papakostas, Lee Baer, Alisabet Clain, Maurizio Fava, David Mischoulon = New Investigator 87 Posters 51 A Case Mix Severity Index for Depression Mark Zimmerman, Rhode Island Hospital 52 How Many Different Ways Do Patients Meet the Diagnostic Criteria for Major Depressive Disorder? Mark Zimmerman, Rhode Island Hospital 53 Randomized Controlled Safety and Efficacy Trials of Lisdexamfetamine Dimesylate for Adults with Moderate to Severe Binge Eating Disorder Susan McElroy, University of Cincinnati College of Medicine James Hudson, M. Celeste Ferreira-Cornwell, Jana Radewonuk, Maria Gasior 54 Prazosin for Nightmares in Patients with Eating Disorders: A Case Series Padmapriya Musunuri, Einstein Medical Center Gibson T. George, Richard Jaffe 55 Effects of Scopolamine on Working Memory Task and Resting Functional Connectivity Using fMRI in Healthy Korean Subjects Brett A. English, PAREXEL International Niki Osimo, Alex Korb, Adam Bazih, Lev Gertsik, Larry Ereshefsky 56 Improving Psychopharmacology Education and Practice: The Quandary of Getting Data and Information to the Teachers Ira Glick, Stanford University School of Medicine Richard Balon, Sidney Zisook 57 Is Pain a Risk Factor for Suicidality as Assessed by the C-SSRS and S-STS? Findings from an Adult Inpatient Psychiatric Sample Ahmad Hameed, Penn State College of Medicine Amanda M. White, Michael Mitchell, Eric A. Youngstrom, Roger E. Meyer, Alan J. Gelenberg 58 A Prospective Open-label Trial of Memantine Hydrochloride for the Treatment of Core Features of Autism Spectrum Disorder in High-functioning Adults Gagan Joshi, Massachusetts General Hospital/Harvard Medical School Janet Wozniak, Ronna Fried, Laura Tarko, Stephannie L. Furtak, Leah Feinberg, Joseph Biederman 88 Posters 59 The Effect of Citicoline Supplementation on Motor Speed and Attention in Adolescent Males Erin McGlade, University of Utah Brain Institute, University of Utah Department of Psychiatry Allison Locatelli, Jennifer DiMuzio, Miho Kizaki, Eri Nakazaki, Toshikazu Kamiya, Deborah Yurgelun-Todd 60 Evaluation of the Implementation of Psychotropic Medication Utilization Parameters for Children and Adolescents in Texas Foster Care M. Lynn Crismon, The University of Texas at Austin James A. Rogers, Alan Shafer, Nina J. Muse 61 How to Collaborate with CRA, from a China Clinical Site Perspective Yifeng Shen, Shanghai Mental Health Center Huafang Li 62 Tolerability and Safety Profile of Aripiprazole Once-monthly in the Treatment of Schizophrenia: A Pooled Analysis from the Safety Database of 11 Completed or Ongoing Trials Ross A. Baker, Otsuka Pharmaceutical Development & Commercialization, Inc. Peter Hertel, Anna-Greta Nylander, Na Jin, Anna Eramo, Ruth Duffy, Robert D. McQuade, Timothy Peters-Strickland 63 Leucocytes Point of Care Measurement in Clozapine Therapy Dan Cohen, Mental Health Organization North-Holland Jan Bogers 64 Early Improvement Predicts Endpoint Response to Lurasidone in Schizophrenia: Pooled Analysis of Five Double-blind Trials Christoph Correll, Hofstra North Shore-LIJ School of Medicine in New York Andrei Pikalov, Jay Hsu, Josephine Cucchiaro, Robert Goldman, Antony Loebel 65 Safety and Tolerability of Cariprazine in Long-term Treatment of Schizophrenia: Integrated Summary of Safety Data Andrew J. Cutler, Florida Clinical Research Center, LLC Henry A. Nasrallah, Yao Wang, Kaifeng Lu, Krisztián Nagy, István Laszlovszky, Suresh Durgam 89 Posters 66 The Effect of Previous Dose of Oral Aripiprazole (10 or 30 Mg/ Day) on the Efficacy and Tolerability of Aripiprazole Oncemonthly: Post-hoc Analyses of Two Double-blind, Randomized, Controlled Trials Anna Eramo, H. Lundbeck A/S Ross A. Baker, Anna-Greta Nylander, Lan-Feng Tsai, Timothy PetersStrickland, Raymond Sanchez 67 Paliperidone Palmitate Delays Relapse and Maintains Functioning in Patients with Stabilized Psychotic and Mood Symptoms of Schizoaffective Disorder Dong-Jing Fu, Janssen Scientific Affairs, LLC Larry Alphs, Jean-Pierre Lindenmayer, Nina R Schooler, Richard B. Simonson, Ibrahim Turkoz, David P. Walling 68 Eszopiclone for Insomnia Treatment in Clinically Stable Patients with Schizophrenia: A Double-blind, Randomized, Placebocontrolled Trial Sinan Guloksuz, Yale University School of Medicine Cenk Tek, Laura Palmese, Andrew Krystal, Pamela DeGeorge, Erin Reutenauer 69 Virtual Reality Functional Capacity Assessment: Progress on the Validation of a Computerized Assessment of Functional Skills Richard S.E. Keefe, NeuroCog Trials, Duke University Stacy Ruse, Vicki G. Davis, Alexandra S. Atkins, Thomas L. Patterson, Meera Narasimhan, Philip Harvey 70 Effect of Long-term Treatment with Lurasidone or Risperidone on Metabolic Syndrome Status in Patients with Schizophrenia John Newcomer, Florida Atlantic University Andrei Pikalov, Kei Watabe, Josephine Cucchiaro, Krithika Rajagopalan, Antony Loebel 71 Aripiprazole Once-monthly for Long-term Maintenance Treatment of Schizophrenia: A 52-week Open-label Study Timothy Peters-Strickland, Otsuka Pharmaceutical Development & Commercialization, Inc. Ross A. Baker, Robert D. McQuade, Anna Eramo, Pamela P. Perry, Brian R. Johnson, Raymond Sanchez, Anna R. Duca 90 Posters 72 Psychometric Properties of the Dynamic Social Cognition Battery (DSCB), a Comprehensive Toolkit for Social Cognition in Patients with Schizophrenia Brian Rothman, ProPhase LLC Anzalee Khan, Luka Lucic, Linda Gao, Mark Opler 73 Prevalence, Healthcare Utilization, and Cost of Patients Dual Diagnosed with Schizophrenia and an Alcohol Use Disorder Bernard L. Silverman, Alkermes, Inc. Jacqueline Zummo, Lauren E. DiPetrillo, Cathy Garabedian 74 Insight into Illness and Uncooperativeness in Chronic Schizophrenia Cynthia Siu, Data Power Ofer Agid, Mary Waye, Gary Remington, Philip Harvey 75 Safety and Efficacy of Aripiprazole Lauroxil: Results from a Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study in Subjects with Acute Exacerbation of Schizophrenia Srdjan R. Stankovic, Alkermes, Inc. Robert Risinger, Yangchun Du, Jacqueline Zummo, Lisa Corey, Bernard L. Silverman, Elliot Ehrich 76 How Does the NSA-4 Compare to the NSA-16? Janet Williams, MedAvante, Inc. Lori M. Garzio, Doug Osman, Danielle Popp 77 Inhaled Loxapine and Intramuscular Lorazepam in Healthy Volunteers: A Randomized, Placebo-controlled Drug-drug Interaction Study Paul P. Yeung, Teva Pharmaceuticals Daniel A. Spyker, James V. Cassella, Randall R. Stoltz 78 Validity Characteristics of the Cognitive Assessment Interview (CAI) in Stable Outpatients with Schizophrenia Robert Goldman, Sunovion Pharmaceuticals, Inc. Joseph Ventura, Cynthia Siu, Antony Loebel 79 The National Pregnancy Registry for Atypical Antipsychotics: Effects of Fetal Exposure on Risk for Major Malformations and Extrapyramidal Symptoms Lee S. Cohen, Massachusetts General Hospital Adele C. Viguera, Kathryn McInerney, Molly Kwiatkowski, Shannon Murphy, Elizabeth Lemon, Sonia Hernandez-Diaz 91 Posters 80 Raving and Depression in Opiate Dependent Mentally Ill Receiving Suboxone and Group CBT Therapy Tanya Alim, Howard University Leslie Adams, Didier Grandjean, Steven Tulin, Elizabeth CarpenterSong, Moria Hipolito, Loretta D. Peterson, William B. Lawson 81 Efficacy and Safety of ABT-126 as a7 Nicotinic Cholinergic Agonist, in Treatment of Cognitive Impairment Associated with Schizophrenia: Results from a Proof Concept Study George Haig, AbbVie, Inc. Jeff Baker, Weining Robieson, Earle Bain, Ahmed A. Othman 92 Author Index 74 81 26, 73, 81 92 70 70 91 92 51 79, 80, 90 34 26, 49 72 32 70 73, 90 87 69 12, 57 85 74 78 87 92 92 78, 79, 89, 90 48 86 88 86 81 71, 74, 75 88 69 70 69 39 71 59 26, 70 70, 82, 88 85 78 70 75 74 89 8, 14, 43, 68 Boucher, Spencer Bowen, Richard Bradshaw, Mark Brennan, Francis X. Brenner, Helena Briggs, Josephine Brimijoin, Stephen Broich, Karl Brown, Delisa Brown, Patricia Brown, Walter Brutsche, Nancy Bryan, Joseph and Kathleen Bryson, Heather Budziszewska, Bogusława Bui, Khanh Bundren, J. Clark Bunker, Mark Burch, Daniel Burdick, Katherine Burghardt, Kyle Burke, Michael A. Busner, Joan Butters, Meryl A. Bymaster, Frank Calabrese, Joseph R. Calarge, Chadi Calderon-Abbo, Jose Camprodon, Joan Camsari, Ulas M. Cantillon, Marc Caroff, Stanley N. Carpenter-Song, Elizabeth Carroll, Kathleen Cassella, James V. Chan, Serena Chang, Kiki Chang, Trina Cheetham, S. Chen, Changzheng Chen, Dalei Chen, Justin Chen, Laishun Chen, Yinzhong Chengappa, Roy K.N. Chen, Yinzhang Cheshire-Kinney, Kim Childress, Ann 93 83 35, 69 69 82 84 13, 57 31 16 84 84 36, 73 87 65 73 71, 74, 75 76 80 25, 74 73 33, 49 26, 70 87 81 85 69 33, 71 50 86 26, 48 81 65 29 92 81 80, 91 86 83 87 77 74 75, 86, 87 87 74 85 49 75 78 69 Author Index Aaronson, Scott T. Abulseoud, Osama Achtyes, Eric Adams, Leslie Addolorato, Giovanni Adler, Lenard Agid, Ofer Alim, Tanya Alpert, Jonathan Alphs, Larry Altemus, Margaret Andreazza, Ana Ansari, Arash Anton, Raymond F. Aoun, Elie G. Atkins, Alexandra S. Atkinson, Sarah D. Atwood, Craig Austin, Christopher Averill, Lynnette A. Bachman, David Badner, Judith A. Baer, Lee Bain, Earle Baker, Jeff Baker, Ross A. Baldwin, David Ball, Susan G. Balon, Richard Barbee, James G. Baskind, Robert Basta-Kaim, Agnieszka Bazih, Adam Beaver, Jessica Bédard, Anne-Claude V. Bencherif, Merouane Ben-Zee, Dror Berk, Michael Bertolino, Alessandro Bhuvaneswaran, Chaya Biederman, Joseph Bilello, John A. Bishop, Jeffrey R. Blair, James Bodkin, J. Alexander Bogan, Richard K. Bogers, Jan Borges, Silvana Author Index Choudhry, Azhar Christine, Mazzucco Chrones, Lambros Citrome, Leslie Clain, Alisabet Clark, Crystal Clarke, Diana E. Clayton, Anita Clayton, Anita H. Coffey, Barbara Cohen, Dan Cohen, Lee S. Compton, Michael T. Corey, Lisa Correll, Christoph Cortese, Bernadette M. Cosgrove, Victoria E. Courbay, Zane Craighead, W. Edward Crismon, M. Lynn Cross, Alan Cucchiaro, Josephine Cunningham, Jacobi I. Curzytek, Katarzyna Cutler, Andrew J. Daly, Ella Daniel, David Datto, Catherine Davidson, Richard J. Davis, Alan T. Davis, Heather L. Davis, Lori Davis, Michael Davis, Vicki G. Deal, Linda Dean, Reginald L. Deaver, Daniel Debelle, Marc DeBoer, Peter DeBonis, Dan Deckersbach, Thilo DeGeorge, Pamela Deligiannidis, Kristina Dellva, Mary Anne Delong, Liz De Luca, Vincenzo DeMartinis, Nicholas 84 69 75 17, 20, 74 87 47, 80 33, 78 75 85 73 78, 89 34, 91 66 91 17, 19, 20, 25, 39, 66, 89 74 61, 71, 83 42 72 89 36, 69 71, 72, 78, 89, 90 85 75 69, 74, 81, 89 69 84 71, 82, 83 31 81 31 16, 19, 25, 33 26, 49 73, 90 77 85 85 78 69 81 17 90 20, 49 86 45 83 84 Dennis, Donn M. Denton, Wayne H. Derbyshire, Katherine L. de Somer, Marc Detka, Jan Detke, Michael Dhossche, Dirk M. DiMuzio, Jennifer DiPetrillo, Lauren E. Dirks, Bryan L. Dodd, Seetal Dragheim, Marianne Drevets, Wayne Du, Yangchun Duca, Anna R. Duda, Weronika Duffy, Farifteh Duffy, Ruth Duhoux, Stephanie Durgam, Suresh Dvergsten, Chris Edwards, Emmeline Efros, Mitchell Ehrhardt, John D. Ehrich, Elliot Ellingrod, Vicki L. English, Brett A. Eramo, Anna Ereshefsky, Larry Escher, Tobie Evins, Eden Eyerman, David Falk, Daniel E. Faraone, Stephen V. Farchione, Tiffany Farr, Gaston Faucett, James Fava, Maurizio Feeley, Louisa Feinberg, Leah Felger, Jennifer Ferguson, Margaret B. Ferreira-Cornwell, M. Fertig, Joanne B. Fiedorowicz, Jess G. Fischer, E. Grace 94 77 70 77 84 71 30, 37, 76 29 89 75, 91 17 71 76, 85, 87 69 49, 84, 91 90 75 78 89 26, 70 72, 78, 89 69 30, 45 73 84 84, 91 70 88 78, 79, 89, 90 76, 88 74 17 85 81 70, 82 17, 19, 25, 50 69 73 16, 17, 20, 50, 58, 66, 73, 75, 76, 84, 87 83 88 26, 85 86 88 81 46 71 Author Index Fleischhacker, W. Flynn, Martina J. Fochtmann, Laura Francis, Andrew Frank, Ellen Freeman, Marlene Friedman, Edward S. Fried, Ronna Frye, Mark A. Fu, Dong-Jing Furmaga, Kevin M. Furtak, Stephannie L. Gabbay, Vilma Gale, Phillip D. Gao, Keming Gao, Linda Garabedian, Cathy Gardner, Kathryn Garneau-Fournier, Jade Garrett, Amy Garzio, Lori M. Gasior, Maria Gastfriend, David Gaynes, Bradley Geibel, Brooke Gelenberg, Alan Gelovich, Mary George, Gibson T. George, Sanju Gerretsen, Philip Gertsik, Lev Gibbons, Robert Gift, Thomas Gilman, Stephen Glick, Ira Gliddon, Emma Goff, Donald C. Goldberger, Celine Goldberg, Joseph Goldman, Robert Golembiowska, Krystyna Gommoll, Carl Goncalves, Vanessa Gosden, J. Grabb, Meg Grandison, Lindsey Grandjean, Didier Grant, Jon E. 78 73 78 29 61, 72 16, 20, 34, 48, 73 37 70, 88 76 90 81 88 73, 75, 86 81 72, 85 71, 79, 91 91 82 83 83 91 77, 88 70 17, 48 30 20, 45, 71, 77, 88 76 88 76 26, 79 88 61, 73 81 87 20, 77, 88 71 51, 66 86 20, 33, 44, 51, 82 89, 91 75 74, 75, 86, 87 83 77 52, 59 19, 25 92 77 Greenberg, William M. Greist, John Grigoriadias, Sophie Grosser, Bernard Grubb, ElizaBeth Guevara, Sara Guloksuz, Sinan Haass-Koffler, Carolina Haig, George Halstead, Scott Hameed, Ahmad Hanina, Adam Hanna, Amira Hanover, Rita Hansel, Tonya C. Haroon, Ebrahim Harper, Linda Harris, Herbert Harris, Margret S. H. Harrison, John E. Harvey, Philip Hassan, Mariam Hassman, Howard Heal, David Heemskerk, Jill Hegarty, Jani Heidi, De Smedt Henry, Michael E. Herman, Barry Hernandez, David Hernandez, Mariely Hernandez-Diaz, Sonia Hert, Marc De Hertel, Peter Hildebrandt, Tom Hill, Lauren Hillefors, Mi Hilsabeck, Robin C. Hipolito, Moria Hosford, David Hough, David Hsu, Jay Hsu, Timothy Hsu, Wei-Chun Hu, Chunling Hudson, James Hummel, Rebecca Hummel, Ruth 95 74 18 6 69 84 87 90 70 92 73 77, 88 39 86 69, 81 86 85 84, 86 82 78 75 90, 91 83 76 77, 86 52 83 69 85 77 78 82 34, 91 79 89 70 15, 19, 25 66 84 92 36, 69 79 89 69, 81 82 76 88 84 84 Author Index Huntley, Kristen Hurt, Stephen W. Hutson, Peter Huz, Ilana Insel, Thomas Ionescu, Dawn F. Iosifescu, Dan V. Iovieno, Nadia Jacobsen, Paula L. Jaffe, Richard Jansen, Joshua F. Jett, John Jha, Amishi Jin, Na Johnson, Amy R. Johnson, Ann E. Johnson, Brian R. Johnson, Joe Johnson, Michael Jones, Warren Joseph, Melissa Joshi, Gagan Joyce, Mark Kamiya, Toshikazu Kane, John Kapczinski, Flavio Kasula, Varun Keefe, Richard Kemp, David Kenna, George Kennedy, James Kennedy, Patrick Kent, Justine Keshavan, Matcheri S. Ketter, Terence Kezic, Iva Khan, Anzalee Khan, Arif Khin, Ni Kim, Sanghyeon Kim, Seonghwan Kim, Sungman King, Bryan H. Kinon, Bruce Kishimoto, Taishiro 30 69 77, 86 87 9, 11, 56, 57 87 51, 60 72 75, 85, 86 88 86 69 30 89 75, 86 81 90 81 87 59 69 88 84 89 20, 39, 46, 66, 67, 78 46 76 18, 73, 90 38, 46 70 83 10, 56 35, 69 78 18, 33, 37, 59, 72, 82, 83 69 71, 79, 91 20, 36, 37, 73, 86, 87 7, 14, 43, 68 79 76 76 59 16, 19 46 Kizaki, Miho Kocsis, James H. Koenig, Aaron Korb, Alex Kott, Alan Kraemer, Helena Kremer, Charlotte Kroger, Hans Krone, Beth Kronstein, Phillip Krupitsky, Evgeny Krystal, Andrew Kubera, Marta Kulkarni, Rajiv Kupfer, David Kurek, Anna Kwiatkowski, Molly Laezza, Fernanda Lally, Níall Landsberg, Wally Lane, Rosanne LaPorte, Scott Lason, Wladyslaw Laszlovszky, István Lauder, Sue Laughren, Thomas Lavigne, Kimberly M. Lawson, William B. Leggio, Lorenzo Leigh-Pemberton, Richard Lemon, Elizabeth Leppink, Eric Leśkiewicz, Monika Leslie, Kimberly R. Levenson, Jessica C. Li, Huafang Li, Sherrie Li, Zhihao Liebowitz, Michael Lim, Kelvin O. Lindenmayer, Jean-Pierre Ling, Walter Lippiello, Patrick Litman, Robert E. Litten, Raye Litten, Raye Z. Locatelli, Allison 96 89 16 26, 85 88 84 18 18 71, 72 70 14, 68 70 90 71, 74, 75 86 16, 18, 52, 57, 72 71 91 82 75 79 69 71, 82, 83 75 72, 78, 89 71 16, 18, 51, 67 86 92 70 75, 84 91 77 71, 74, 75 74 72 79, 89 83 85 35, 47, 60, 69, 81 30 79, 90 70 69 72 16, 31 32, 38, 81 89 Author Index Loebel, Antony Loewy, Rachel Loft, Henrik Lombard, Jay Lophaven, Søren N. Loskie, Eileen Lucic, Luka Luckenbaugh, David Lu, Kaifeng Mackin, Scott Maes, Michael Mahableshwarkar, Atul R. Mahalchick, Lucy Ma, Kaizong Malhotra, Anil Maneru, Cristina Manji, Husseini Mao, Lian Mao, Yongcai Marangell, Lauren B. Marc, Ceusters Marchant, Barrie K. Marder, Steve Marshall, Kay Marshall, Randall D. Martinez, James M. Masand, Prakash Mathew, Sanjay Mathews, Maju Mattes, Jeffrey Matthews, Mark Mattingly, Gregory Matyas, Gary R. Mavissakalian, Matig McCracken, James T. McElroy, Susan McEvoy, Joseph P. McGlade, Erin McInerney, Kathryn McKinney, Anthony McQuade, Robert D. Memisoglu, Asli Menosky, Angela Merritt-Davis, Orlena Meyer, Roger E. Michelson, David Miller, Andrew H. 71, 72, 78, 79, 82, 83, 89, 90, 91 67 76 82 75 77 71, 91 75, 87 72, 78, 89 50 75 75, 76, 85, 86 78 71 16, 18 83 8, 15, 16, 55, 56 80 78 86 69 81 16, 18 81 60, 69, 75, 84 86 76 60, 76, 87 74, 75, 86, 87 77 61 79 31 47 59 58, 77, 88 47 89 91 69 89, 90 70, 75, 84 84 74 77, 88 16 85 Millet, Robert Mischoulon, David Miskowiak, Kamilla Miskowiakm, Kamilla Mitchell, Michael Mohammad, Othman Molpus, Robert Monti, Louis Montoya, Ivan Mościcki, Eve K. Mullen, Jamie Murphy, Shannon Murrough, James W. Muse, Nina J. Musunuri, Padmapriya Nagy, Krisztián Nakajima, Shinichiro Nakamura, Richard Nakazaki, Eri Narasimhan, Meera Narrow, William Nasrallah, Henry A. Nations, Kari Neill, Joanna C. Nelson, Craig Nenov, Miroslav Newcomer, John Newcorn, Jeffrey Nguyen, Theresa Niciu, Mark Nicol, Ginger E. Nielsen, Rebecca Z. Nierenberg, Andrew Nimgaonkar, Vishwajit Nolan, Neal Norquist, Grayson Nueten, Luc Van Nugent, Allison C. Nugter, Annet Nunez, Rene Nylander, Anna-Greta O’Brien, Catherine O’Connor, Joann Odlaug, Brian L. Olsen, Christina Kurre O’Malley, Stephanie Ondrus, Matej O’Neill, James 97 76 73, 87 26 48 77, 88 72 84 69 19 34, 78 83 91 60 89 88 72, 89 26 13, 57 89 90 33, 34, 78 89 84 81 18, 50 82 20, 71, 90 70 72 26, 87 71 87 16, 37, 67 78 87 67 69 75 78 74, 75, 86, 87 89, 90 69, 81 74 77 75 16 77 78 Author Index Opler, Mark Oshibuchi, Hidehiro Osimo, Niki Oslin, David Osman, Doug Osser, David Othman, Ahmed A. Paige, Staudenmaier Paik, Jong-Woo Palfi, Sandor Palmese, Laura Palo, William Pangallo, Beth A. Panova-Elektronova, Neli Papakostas, George I. Parkinson, Amy Pathak, Sanjeev Patkar, Ashwin Patterson, Thomas L. Pearlstein, Jennifer Pedraza, Juan D. Pendergrass, J. Cara Perez-Rodriguez, M. Perry, Pamela P. Persson, Jennie K. Petersen, Timothy Peterson, Loretta D. Peters-Strickland, Timothy Petrakis, Ismene L. Petrides, Georgios Phillips, Debra Phillips, Katharine Pikalov, Andrei Pilar, Lim Piser, Tim Plovnick, Robert M. Pooley, James Popescu, Marlene Popp, Danielle Posener, Joel Pothier, David D. Potter, William Prasad, Konasale M. Price, Blair Priest, Jacob B. Prisciandaro, James J. Prow, M. 71, 79, 91 26 88 38 91 72 92 83 76 83 90 85 86 82 59, 72, 85, 87 83 76, 87 76 90 83 70 84 26, 49 90 77 60 92 49, 78, 89, 90 38 29 78, 79 18, 19, 25 33, 71, 78, 79, 82, 83, 89, 90 69 76 78 73 77 91 76 79 16, 52 78 76 70 84 77 Quirk, Michael Rabinowitz, Jonathan Radewonuk, Jana Rajagopalan, Krithika Raju, Karishma Rapaport, Mark H. Rapoport, Stanley Rappaport, Bob A. Rauh, Philip Regulska, Magdalena Reilly, James L. Reimherr, Frederick W. Reimherr, Kathleen Rele, Shilpa Remington, Gary Ressler, Kerry Reutenauer, Erin Reynolds, Charles F. Richards, Erica M. Riesenberg, Robert Rishton, Melanie Risinger, Robert Robieson, Weining Rodriguez, Stephen Rogers, James A. Romano, Steve Rorick-Kehn, Linda Rose, Andrew C. Rosenbaum, Jerrold Rosenberg, Leon I. Rothman, Brian Rowley, Helen L. Roybal, Donna Rubin, Jonathan Rubin, Robert A. Runion, Jennifer Ruse, Stacy Rush, A. John Ruth, Adam Ruvuna, Francis Ryan, Megan L. Ryan, Neil Ryan, Patrick Sachs, Gary S. Sahoo, Saddichha Sajatovic, Martha Salman, Ester Saltz, Bruce 98 76 30, 35 88 82, 83, 90 71 15, 17, 19, 20, 25 71 17 84 75 78 81 81 76 91 72 90 85 87 87 77 91 92 78, 80 89 17, 18 82 81 18 76 71, 79, 91 86 26, 83 35 85 74 90 5, 25 74 74 81 18 79 81, 83 50 18 81 18 Author Index Sanacora, Gerard Sanchez, Raymond Sarampote, Chris Sarma, Kaushik Saunders, Erika Savitz, Jonathan Scahill, Lawrence Schaefer, Eric Schneider, Lon S. Schooler, Nina R. Schronen, Juan Schulz, Kurt Schweiger, Julia A. Scott, Rachel Sears, Peter Sedway, Jan Segreti, Anthony Serenko, Michael Shafer, Alan Shavkunov, Alexander Shay, Michelle Shelton, Richard Shen, Yifeng Shiromani, Priyattam Shurtleff, David Silk, Jennifer Silva, Robert Silverman, Bernard L. Simeonova, Diana Simon, Greg Simonson, Richard B. Simpson, Tracy Singareddy, Ravi Singh, Jaskaran Siu, Cynthia Skolnick, Phil Slusarczyk, Joanna Smart, LeAnn D. Smit, Andries Smith, Katie Smith, Purvi K. Smith, Robert C. Sofuoglu, Mehmet Sorensen, Per Spencer, Thomas Spyker, Daniel A. Stang, Paul 60, 66, 76, 87 78, 90 52 71, 72 71 46 59 71 65 17, 19, 25, 39, 46, 47, 67, 90 87 70 71 82 74 83 69 86 89 82 71, 82 18, 51, 76, 85 79, 89 74 30 61 71, 72 70, 84, 91 26, 72 45, 67 90 38 74 35, 60, 69 82, 83, 91 11, 17, 31, 57 74, 75 73, 81 78 85 83 80 81 32 70 80, 91 79 Stankovic, Srdjan R. Starr, H. Lynn Staudenmaier, Paige Steans, Tammy A. Stein, Mark Stevenson, James M. Stoltz, Randall R. Stout, Robert L. Strachan, Martha Stroescu, Ioan Su, Hong-Lin Suppes, Trisha Surman, Craig Sutor, Shari Swartz, Holly Sweeney, John A. Swift, Robert Tandon, Rajiv Tanenbaum, Russell Tang, Xiongwen Targum, Steven D. Tarko, Laura Taub, Floyd Tauscher-Wisniewski, Sitra Tek, Cenk Thase, Michael Thurmond, Linda M. Tiwari, Arun K. Tocco, Michael Todtenkopf, Mark S. Tohen, Mauricio Toler, Steven Tolliver, Bryan Tomlinson, Anneka Traeger, Lara Trinh, Nhi-Ha Trivedi, Madhukar Tsai, Lan-Feng Tulin, Steven Turkoz, Ibrahim Turncliff, Ryan Tye, Susannah J. Uhde, Thomas W. Valjakka, Risto Vallejo, Ana I. Vanover, Kimberly E. Varghese, Sajoy P. 99 91 80 83 81 70 78 91 32 74 73 76, 87 71 70 76 15, 19, 25, 61, 72 78 70 78 84 74 36, 50, 84 70, 82, 88 85 66 90 15, 17, 20, 44, 51, 75, 76 85 83 79 85 37 69 84 48, 81 87 26, 87 46, 75, 76 78, 79, 90 92 90 75 76 74 77 75, 86 36 74 Author Index Ventura, Joseph Viguera, Adele C. Vijapura, Sagar A. Vincent, John B. Vitiello, Ben Volk, Stephen Vornov, James Voss, Erica Wagner, Ann Wagner, Karen Walker, Adam Walker, Rosemary Walling, David P. Wang, Chao Wang, Dana Wang, Yao Warnock, Julia “Jill” K. Warren, Kenneth Watabe, Kei Waye, Mary Weber, Wendy Weiden, Peter J. Weingard, Kerri Welsh-Bohmer, Kathleen Wender, Paul H. Wesnes, Keith White, Amanda M. White, Stuart F. Wilens, Timothy Williams, Janet Winchell, Celia Wirth, Robert Witkiewitz, Katie Woods, Sarah B. Wood, Terri Woolwine, Bobbi Wozniak, Janet Wright, Jason Wright, Suzin M. Wu, Allen Xu, Jane Yaroslavsky, Yury Yeung, Albert Yeung, Paul P. Yingling, Michael D. Yonkers, Kimberly Youngstrom, Eric A. Yurgelun-Todd, Deborah Zai, Clement Zai, Gwyneth Zajecka, John Zarate, Carlos 91 91 87 83 17, 19, 67 78 76 79 59 19, 20 76 72 90 76 72 89 80 12, 57 82, 90 91 45 45 73 65 81 65, 76 77, 88 70 35, 69, 81 Zimmerman, Mark Zisook, Sidney Zukin, Stephen Zummo, Jacqueline Zumpano, Laura 100 19, 30, 77, 91 7, 14, 43, 68 77 32 70 84 85 82, 88 71, 82 85 78 79 74 87 80, 91 71 19, 81 77, 88 89 26, 83 26, 48 87 15, 17, 19, 35, 66, 75, 87 73, 88 20, 88 74, 78 70, 91 84 Save the Date: 2015 ASCP Annual Meeting June 22-25, 2015 Miami Beach, Florida www.ascpp.org ASCP Executive Office 5034A Thoroughbred Lane Brentwood, Tennessee 37027 phone: 615-649-3085 fax: 888-417-3311 email: info@ascpp.org