General Chemistry Lab - Seattle Central College
advertisement

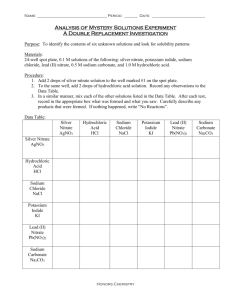
General Chemistry Lab Experiment 6 Types of Chemical Reaction Introduction Most ordinary chemical reactions can be classified as one of five basic types. The first type of reaction occurs when two or more substances react to form a single compound. This type is called a combination reaction. A + Z ´ AZ A second type of reaction occurs when a single compound breaks down into two or more simpler substances, usually by the application of heat. This type is called a decomposition reaction. AZ ´ A + Z A third type of reaction occurs when one element displaces another element from a compound or aqueous solution. For this reaction to occur, the element that is replaced must be lower in the activity series. This type is called a single-replacement reaction. A + BZ ´ AZ + B A fourth type of reaction occurs when two substances in aqueous solution switch partners; that is, an anion of one substance exchanges with another. This type is called a double-replacement reaction. AX + BZ ´ AZ + BX A fifth type of reaction occurs when an acid and a base react to form a salt and water. This type is called a neutralization reaction. HX + BOH ´ BX + HOH Notice the hydrogen ion in the acid neutralizes the hydroxide ion in the base to form water. If water is written as HOH, the neutralization is more obvious and the equation may be easier to balance. In this experiment, we will carefully observe and record evidence for a chemical reaction. Evidence for a reaction may include any of the following: (1) a gas is produced; (2) a precipitate is formed; (3) a color change is observed; (4) an energy change is noted. In order to describe the reaction, we use various symbols in the chemical equation. Table 13.1 lists some of these. Table 1 Symbols in Chemical Equations Symbol___ ´ + U NR (s) (f) (g) (aq) ___Translation produces, yields (separates reactants from products) added to, reacts with (separates two or more reactants or products) heat (written above —>) no reaction (written after —>) solid or precipitate liquid gas aqueous solution 1-9 In order to write an equation, it is necessary to predict the products from a given reaction. Initially, this is a difficult task. To aid you in writing equations, word equations are supplied for each reaction. However, it is necessary to translate the word equations into balanced chemical equations. The following examples will illustrate. A. Conbination Reaction iron(s) + oxygen ´ iron (III) oxide 4Fe + 3O2 ´ 2Fe2O3 B. Decomposition Reaction lithium hydrogen carbonate(s) ´ lithium carbonate(s) + steam(g) + carbon dioxide(g) 2 LiHCO3(S) ´ Li2C03(s) + H20(g) + CO2(g) C. Single-Replacement Reaction tin(s) + hydrochloric acid(aq) ´ tin(II) chloride(aq) + hydrogen(g) Sn(s) + 2 HCl(aq) ´ SnCl2(aq) + H2(g) ___________________________________________________________________________ D. Double-Replacement Reaction potassium carbonate(aq) + calcium chloride(aq) ´ calcium carbonate(s) + potassium chloride(aq) K2C03(aq) + CaCl2(aq) ´ CaCO3(s) + 2 KCl(aq) ___________________________________________________________________________ E. Neutralization Reaction — nitric acid(aq) + barium hydroxide(aq) ´ barium nitrate(aq) + water 2 HNO3(aq) + Ba(OH)2(aq) ´Ba(NO3)2(aq) + 2HOH(l) _________________________________________________________________________ PROCEDURE General Directions: For Procedures A-E, record your observations in the Data Table. A. A. Combination Reactions - Instructor Demonstration 1. Hold a 2 cm strip of magnesium ribbon with crucible tongs and ignite the metal in a hot burner flame. B. Decomposition Reactions 1. Put a few crystals of copper(II) sulfate pentahydrate in a dry test tube. Grasp the test tube with a test tube holder or a two prong clamp. Heat the side of the test tube with a burner. Use a soft blue flame. Note the color change and observe the inside wall of the test tube. 2. Add sodium hydrogen carbonate (baking soda) into a 250 mL Erlenmeyer flask so as to sparsely cover the bottom. Support the flask on a ring stand using a wire gauze. a. Hold a flaming splint in the mouth of the flask for 10 seconds. Does the splint continue to burn or does the flame blow out. b. Heat the flask strongly with the laboratory burner until moisture is observed on the side of the flask; quickly hold a flaming splint in the mouth of the flask and record how long it burns. 2-9 C. Single-Replacement Reactions 1. Put 20 drops of silver nitrate solution into a test tube and add a small piece of copper wire. Allow a few minutes for reaction and then record your observation. 2. Put 20 drops of hydrochloric acid into a test tube and add a small piece of magnesium metal. Record your observation. 3. Put 20 drops of distilled water into a test tube and add a small piece of calcium metal. Record your observation. D. Double-Replacement Reactions 1-3. Put 10 drops of silver nitrate, copper(II) nitrate, and aluminum nitrate solutions into separate test tubes #1-3. Add a few drops of ammonium carbonate solution into test tubes #1, #2, and #3. Observe and record your observations. 4-6. Put 10 drops of silver nitrate, copper(II) nitrate, and aluminum nitrate solutions into separate test tubes #4-6. Add a few drops of sodium phosphate solution into test tubes #4, #5, and #6. Observe and record your observations. E. Neutralization Reactions 1. Put 10 drops of nitric acid, sulfuric acid, and phosphoric acid into separate test tubes # 1-3. Add one drop of phenolphthalein into each of the test tubes. Add drops of dilute sodium hydroxide solution into test tube #1 until a permanent color change is observed. Note: Phenolphthalein is an acid-base indicator that is colorless in acidic and neutral solutions and pink in basic solutions. 2. Add drops of dilute sodium hydroxide solution into test tube #2 until a permanent color change is observed. 3. Add drops of dilute sodium hydroxide solution into test tube #3 until a permanent color change is observed. 3-9 4-9 Name ________________________________________________________________ DATA TABLE Procedure _________________________ Evidence for Reaction A. Combination Reactions - Instructor Demonstrations 1. Mg + O2 ´ __________________________________________________ B. Decomposition Reactions U 1. CuSO4.5H2O ´ __________________________________________________ U 2. NaHCO3 ´ __________________________________________________ C. Single-Replacement Reactions 1. Cu + AgNO3 ´ __________________________________________________ 2. Mg + HC1 ´ __________________________________________________ Ca + H2O ´ __________________________________________________ 3. D. Double-Replacement Reactions 1. AgNO3 + (NH4)2CO3 ´ ____________________________________________ 2. Cu(NO3)2 + (NH4)2CO3 ´ ____________________________________________ 4. A1(NO3)3 + (NH4)2CO3 ´ ____________________________________________ 5. AgNO3 + Na3PO4 ´ ____________________________________________ 6. Cu(NO3)2 + Na3PO4 ´ ____________________________________________ 7. A1(NO3)3 + Na3PO4 ´ ____________________________________________ E. Neutralization Reactions 1. HNO3 + NaOH ´ ____________________________________________ 2. H2SO4 + NaOH ´ ____________________________________________ 3. H3PO4+ NaOH ´ ____________________________________________ 5-9 Translate Each Word Equation into a Balanced Chemical Equation A.Combination Reactions - Instructor Demonstrations 1. magnesium(S) + oxygen(g) ´ magnesium oxide(s) A. Decomposition Reactions 1. copper(II) sulfate pentahydrate(S) ´ copper(II) sulfate(S) + water(g) 2. sodium hydrogen carbonate (s) ´ sodium carbonate(S) + water(g) + carbon dioxide(g) C. Single-Replacement Reactions 1. copper(s) + silver nitrate (aq) ´ copper(II) nitrate (aq) + silver(s) 2. magnesium(S) + hydrochloric acid(aq) ´ magnesium chloride (aq) + hydrogen (g 3. calcium(s) + water(l) ´ calcium hydroxide (s) + hydrogen (g) D. Double-Replacement Reactions silver nitrate(aq) + ammonium carbonate(aq) ´silver carbonate(S) + ammonium nitrate(aq) 1. copper(II) nitrate(aq) +ammonium carbonate(aq) ´ copper(II) carbonate(s) +ammonium nitrate(aq) 2. aluminum nitrate(aq) +ammonium carbonate(aq) ´ aluminum carbonate(S) + ammonium nitrate(aq) 3. 4. silver nitrate(aq) + sodium phosphate(aq) ´ silver phosphate(S) + sodium nitrate(aq) 5. copper(II) nitrate (aq) + sodium phosphate(aq) ´copper(II) phosphate(S) + sodium nitrate(aq) 6. aluminum nitrate(aq) + sodium phosphate(aq) ´ aluminum phosphate(S) + sodium nitrate(aq) E. Neutralization Reactions 1. nitric acid (aq) + sodium hydroxide (aq) ´ sodium nitrate(aq) + water 2. sulf uric acid (aq) + sodium hydroxide (aq) ´ sodium sulfate (aq) + water 3. phosphoric acid (aq) + sodium hydroxide (aq) ´ sodium phosphate (aq) + water 6-9 NAME ____________________ DATE _____________ POSTLABORATORY ASSIGNMENT 1. Provide the chemical formula for the following substances produced during the experiment. Refer to pages 148-149 for the substances produced from the chemical reactions. (a) the white smoke produced from reaction A.1 __________ (b) the colorless liquid produced from reaction B.1 __________ (c) the flame-extinguishing gas produced from reaction B.2 __________ (d) the gray solid produced from reaction C.I __________ (e) the colorless gas produced from reaction C.2 __________ (f) the yellow ppt produced from reaction D.I __________ (g) the blue ppt produced from reaction D.2 __________ (h) the white ppt produced from reaction D.3 __________ 3. Convert the following word equations to balanced chemical equations, (a) copper metal (S) + oxygen(g) ´ copper(II)oxide (s) (b) iron(m) carbonate(S) ´ iron(in) oxide(S) + carbon dioxide(g) (c) sodium metal (S) + water(l) —´ sodium hydroxide (aq) + hydrogen (g) (d) aluminum metal (s) + sulfuric acid (aq) ´ aluminum sulfate (aq) + hydrogen (g) (e) copper(II) sulfate (aq) + lithium chromate(aq) ´ copper(II) chromate(s) + lithium sulfate(aq) (f) acetic acid (aq) + barium hydroxide(aq) ´barium acetate(aq) + water 7-9 8-9 Name:_____________________________________________________________________ PRELABORATORY ASSIGNMENT 1. In your own words, define the following terms: activity series - aqueous solution - catalyst - precipitate (ppt) - product - reactant - 2. Explain the meaning of the following symbols: ´, +, A, NR, (s), (l), (g), (aq) 3. List four observations that are evidence of chemical reaction. 4. What color is phenolphthalein indicator in (a) an acidic solution? (b) a basic solution? 9-9