Quiz 4
advertisement
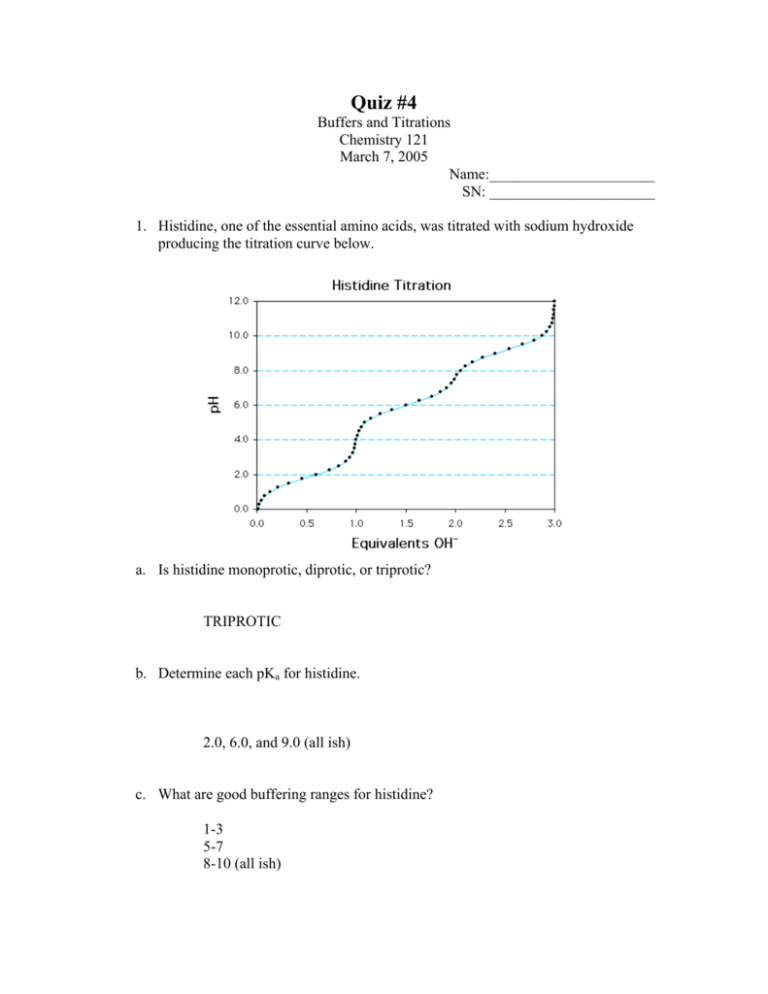
Quiz #4 Buffers and Titrations Chemistry 121 March 7, 2005 Name:______________________ SN: ______________________ 1. Histidine, one of the essential amino acids, was titrated with sodium hydroxide producing the titration curve below. a. Is histidine monoprotic, diprotic, or triprotic? TRIPROTIC b. Determine each pKa for histidine. 2.0, 6.0, and 9.0 (all ish) c. What are good buffering ranges for histidine? 1-3 5-7 8-10 (all ish) 2. Calculate the pH of a solution containing 0.050 M CH3CO2H (acetic acid, Ka = 1.8 x 10-5) and 0.150 M CH3CO2Na (sodium acetate) From pH = pKa + log (base/acid) you get 5.22 3. What would the pH be when 0.050 moles of gaseous HCl is added to 1 L of the above solution? In that case you would have converted 0.050 mol of the acetate to acetic acid using the HCl, making the concentration of the acid equal to the concentration of the base, so the pH = the pKa





![Biochemistry 311 Problem Set: pH and Buffer 1. Calculate the [H+] of](http://s3.studylib.net/store/data/008387530_1-6ccd922b6832805c7f43502d9af7b39b-300x300.png)