Lab # 7: Electron Probability

Name:
Lab Partner(s):
Per: Date
Lab # 7: Electron Probability
Accelerated Chemistry 1
Problem
: How well can we model an orbital with a “dartboard”?
Introduction
What does the term “probability” really mean? The Webster definition is, “the chance that an event will occur.” According to modern atomic theory, we cannot be sure of the exact location of electrons in an atom; due to an electron’s size and speed it is impossible to pinpoint an exact location at any given time. We then must discuss the “location” of an electron in terms of probability instead of an exact position in order to develop a model of the atom. We predict that electrons will be relatively close to the nucleus (because the electrons are negatively charged and the nucleus is positively charged). This is why a dartboard is a good analogy for an atomic orbital. In this activity you will construct and analyze a probability map using a marble and target to better understand the probability map that is an atomic orbital.
Materials
Target circles
Procedure
Marble Carbon paper
1.
Place the carbon paper with the carbon side toward the target circles; lay the two sheets on the floor.
2.
Drop the marble from shoulder height, trying to hit the center of the target. Catch the marble on the rebound, before it hits the floor again.
3.
Repeat this procedure 99 times for a total of 100 drops.
4.
Count the number of hits in each ring and record this number.
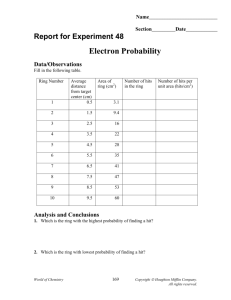
Data/Observations
Complete the following table.
Ring Number
Average distance from target center
(cm)
Area of ring (cm 2 )
Number of hits in the ring
Number of hits per unit area
(hits/cm 2 )
1 0.5 3.1
4
5
2
3
1.5
2.5
3.5
4.5
9.4
13
22
28
8
9
6
7
10
AccelLab7-ElectronProb
5.5
6.5
7.5
8.5
9.5
35
41
47
53
60
1
Analysis and Conclusions
[ hint: questions 1-3 and 5-7 do not relate to electrons in an atom!
]
1.
Where is the density of marble hits the greatest? Why is this?
2.
Density of hits: construct a graph of hits per unit area vs. average distance from the center; include a best-fit line.
3.
What does the graph of the hits per unit area vs. average distance represent? Account for its shape.
4.
Compare your density of hits graph with the hydrogen 1 s electron probability density. Why is the probability of finding an electron at a particular position greatest near the nucleus?
5.
Which is the ring with the highest probability of finding a hit (i.e. which ring has the most hits)?
6.
Radial probability distribution: construct a graph of the number of hits vs. average distance from the center; include a best-fit line.
7.
What does the graph of the number of hits vs. average distance represent? Account for its shape.
8.
Are the maxima of your two graphs the same? Explain.
9.
What is the radius of the 90% probability circle for your marble drops?
10.
How would increasing the number of drops affect your results?
AccelLab7-ElectronProb 2
1 2 3 4 5 6 7 8 9 10
AccelLab7-ElectronProb 3