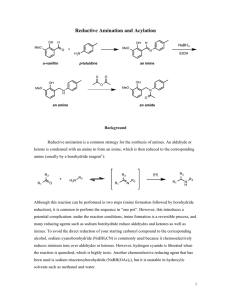
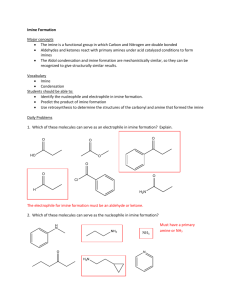
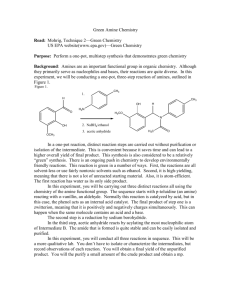
Reductive Amination Procedure
advertisement

REDUCTIVE AMINATION: Three Easy Pieces Adapted from J. Chem. Ed.; Kim Touchette: Bard College, Annandale-on-Hudson, NY CH3 O CH3 H H O H NH2 OCH3 - H2 O Step 1 O CH3 OH H3 CO N 1. NaBH4 H 2. AcOH/AcOAc OCH3 Step 2 & 3 N C H3 C O Reductive amination is usually described as a one-pot procedure in which an aldehyde or ketone reacts with ammonia or an amine to form an imine, which is selectively reduced as it is formed. Hydrogen over nickel or a weakened hydride donor (NaBH3CN, NaBH(OAc)3) is commonly used to reduce the imine as these reagents are slow to reduce the carbonyl compound. In this experiment we will react ortho-vanillin with para-toluidine to generate an imine. The reaction occurs between the two solids in a solvent free reaction. The imine is subsequently reduced with sodium borohydride to the amine, followed by acetylation to afford a solid amide derivative. The entire reaction sequence is performed in an open beaker. Experimental Procedure: A. Synthesis of 2-methoxy-6-(p-tolyliminomethyl)-phenol: Imine formation Tare a 250 mL beaker and then add 0.76 grams (5 mmol.) of ortho-vanillin. Record the total mass of the beaker plus the ortho-vanillin. Using weighing paper, accurately weigh an equivalent amount of paratoluidine (0.535 grams, 5 mmol.) and add this to the beaker. Observe this mixture for a few minutes and record what is happening. Using a heavy glass-stirring rod or a mortar and pestle, mix and grind the solids until they become a homogeneous dry powder. Weigh the beaker and record the mass. Determine the percent yield. Remove a small sample of this material for NMR analysis. Compare the features of your spectrum with those of the starting materials. B. Synthesis of N-(2-hydroxy-3-methoxybenzyl)-p-methylaniline: Reduction of the Imine Add about 15 mL of 95% ethanol and a stir bar to the beaker containing your imine product and stir the mixture to partially dissolve the imine. Weigh out approximately 0.1 grams of sodium borohydride and slowly add this to the beaker in small increments with continued stirring. Record all observations and explain what is occurring in the reaction. C. Synthesis of N-(2-hydroxy-3-methoxybenzyl)-N-p-tolylacetamide: Acetylation of the Amine Add 2 mL of acetic acid to the amine to destroy the excess borohydride and to neutralize the phenoxide ion. Add 2 mL of acetic anhydride and gently warm the solution on a hot plate for 5-10 minutes (do not boil). Last edited 10/24/12 1 Turn the heat off and allow the reaction to slightly cool. Stir the solution fairly rapidly while slowly adding 75 mL of water. Continued stirring should leach out the alcohol and acetic acid causing the amide product to precipitate. Cool the mixture in an ice bath, collect the solid and rinse with a small amount of cold water. Allow it to air-dry until next week and then analyze your product by melting point. D. Preparing an NMR Sample Each student will prepare an NMR sample of either the imine intermediate or the final amide. Listen carefully to your TA, as you will need to know which compound to use as your sample, whether to prepare a sample for 13C NMR or 1H NMR and what your sample number should be. Record this information in your lab notebook. You will also be sharing data with a lab partner, so that you have access to a proton and carbon NMR of the same compound. Record your partner’s sample number as well so you can access their data. ~15 mg compound and dissolve it in 0.6 mL CDCl3. Make sure the sample is clear (it will be orange but translucent) and contains no solids. Report/Post-lab questions: 1. Determine your overall percent yield for the three step synthesis and characterize your final product by melting point. 2. The structure of capsaicin, the pungent ingredient in red pepper or capsicum annuum, is shown below. Suggest a multi-step synthetic scheme analogous to the sequence used in this experiment to prepare capsaicin. O HO CH2NHC(CH2)4CH=CHCH(CH3)2 H3CO Last edited 10/24/12 2