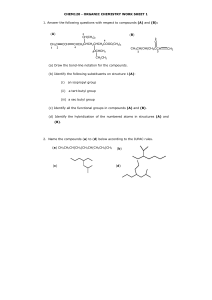
Draw line structures for the following compounds
advertisement

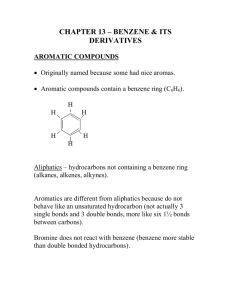
1) Draw line structures for the following compounds: a) b) c) d) e) f) 2,2,4-Trimethylhexane 2,2-Dimethylpropane trans-2-Methyl-3-hexene 3-Ethyl-3-methyl-1-pentyne 2-Methyl-1-butene 2,3,7-trimethyloct-2-ene 2) Write IUPAC names for the following alkanes: 3) Explain why each of the following is an incorrect IUPAC name. Write the correct IUPAC name for the compound. a) b) c) d) e) f) g) h) i) j) 1,3-Dimethylbutane 4-Methylpentane 2,2-Diethylbutane 2-Ethyl-3-methylpentane 2-Propylpentane 2,2-Diethylheptane 2,2-Dimethylcyclopropane 1-Ethyl-5-methylcyclohexane 2-Ethyl-2-hexene 4-Hexyne 4) How many isomers (same molecular formula but different structural formulae) are possible for C5H12? Draw them. 5) Draw the two isomers for C3H6 6) Which of these alkenes show cis-trans isomerism? For each that does, draw structural formulas for both isomers. a) b) c) d) e) f) 1-Hexene 2-Hexene 3-Hexene 2-Methyl-2-hexene 3-Methyl-2-hexene 2,3-Dimethyl-2-hexene 7) What reagents and/or catalysts are necessary to bring about each conversion? 8) Show what product is formed when 3-hexyne is hydrogenated with excess hydrogen using a Pd/C catalyst. What is the product when using a Lindlar catalyst? 9) Write down the structural formula for the product of each of the following reactions. Include all reagents and catalysts: a) b) c) d) Benzene to nitrobenzene Benzene to bromobenzene Benzene to chlorobenzene Benzene to toluene 10) Show by a two-step process how one might prepare the following compounds from benzene. Include all reagents and catalysts. Br Cl NO2 Cl Cl Br Br 11) Draw the structural formula for the products formed when each compound is treated with K2Cr2O7/H2SO4 (called Jones reagent, an oxidizing agent). If no reaction takes place, indicate so. O O H3C OH H 12) From what carboxylic acid and alcohol is each of the following esters derived? O O O CH3 H3C CH3 O OH 13) 2-Pentanol is chiral, but 3-pentanol is not. Explain 14) Identify the chiral stereocentres in the following molecules H N F 3C O O N CH3 O O H F 15) Write a general equation for the formation of polyvinyl chloride from vinyl chloride and show a portion of the polymer containing three monomer units. 16) From the following monomers, show a portion of the copolymer containing three monomeric units. Indicate the repeat unit of the copolymer. 17) What is the difference between thermosetting and thermoplastic polymers? Give an example of each. 18) Describe how natural rubber can be made more useful. How has this changed its properties?