Nuclear Chemistry
advertisement

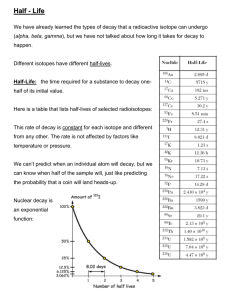
21.1 Lab Activity Nuclear Chemistry North High Chemistry Name Hour Radioactive Decay of Candium How long will it take for a sample of Eminemium to completely transmutate into a new element? Procedure: 1. Count and record the total number of m&m’s that you start with. 2. Place all the atoms of Eminemium into the cup. 3. Record your “Start time” to the nearest minute. 4. Cover the cup and gently shake for a few (3-7) seconds. 5. Gently pour out the candy onto a plate or onto a sheet of paper. 6. Count the number of pieces with the print side up and record the number in 1) “Number of Undecayed Atoms.” 7. Return the “Undecayed Atoms” to the cup. 8. Count the number of pieces with the print side down and record the number in 1) “Number of Decayed Atoms.” 9. Consume or stash the “decayed” atoms. 10. Gently shake the cup again for a few seconds. 11. Repeat steps 5-9 for Half-life. 12. Continue shaking, counting and consuming until all of the atoms have decayed. 13. Record your “End time” to the nearest minute. 14. Determine the “Average half-life in seconds” by finding the change in end time vs. start time in minutes and then multiply your answer by 60 to convert to “Total time in seconds”. Record your answer. Next, divide the “Total time in seconds” by the total number of Half-lives to find the “Average half-life” and record it. 15. Use the data to graph of the Number of Undecayed Atoms vs. Total Time in Seconds on the backside of this lab sheet. Data: Total number of m&m’s your group started with __________ (1st point on graph) Start time in minutes _________ End time in minutes __________ Change in time (end - start time) in minutes = ________ X 60 = __________Total time in sec. Total time in sec. _______ Half-life 1) 2) 3) 4) 5) 6) 7) 8) 9) 10) 11) 12) / Total number of half-lives = ________ Average half-life in sec. Number of Undecayed Atoms Number of Decayed Atoms Graph of the Number of Undecayed Atoms vs. Total Time 150 140 130 120 110 90 80 70 60 50 40 30 20 10 0 Number m&m’s started with Number of Undecayed Atoms 100 1st ½ life TOTAL TIME 2nd ½ life TOTAL TIME 3rd ½ life TOTAL TIME 4th ½ life TOTAL TIME 5th ½ life TOTAL TIME 6th ½ life TOTAL TIME 7th ½ life TOTAL TIME 8th ½ life TOTAL TIME 9th ½ life TOTAL TIME ______ ______ ______ ______ ______ ______ ______ ______ ______ Total Time (sec.) Questions: 1. How would a longer half-life affect the shape of the graph? 2. Using the graph, how many m&m’s would you have at 4 ½ half-lives? ____________ 3. If you had started with twice as many m&m’s, how many more half-lives would it have taken, on average, for your “sample” to totally decay? ____________ 4. If you have four times as much radioactive material with a half-life of 10 years, how many, on average, more years would it take to totally decay? ____________ 5. If you had one million “Skittlium” with a half-life of 100 seconds, how long would it take to completely decay? ____________ 6. Considering the average length of Eminemium’s half-life, would you consider it a long or short half-life ____________ and what are the advantages and disadvantages?