Chem 223 Lab Borohydride Reduction of Camphor
advertisement
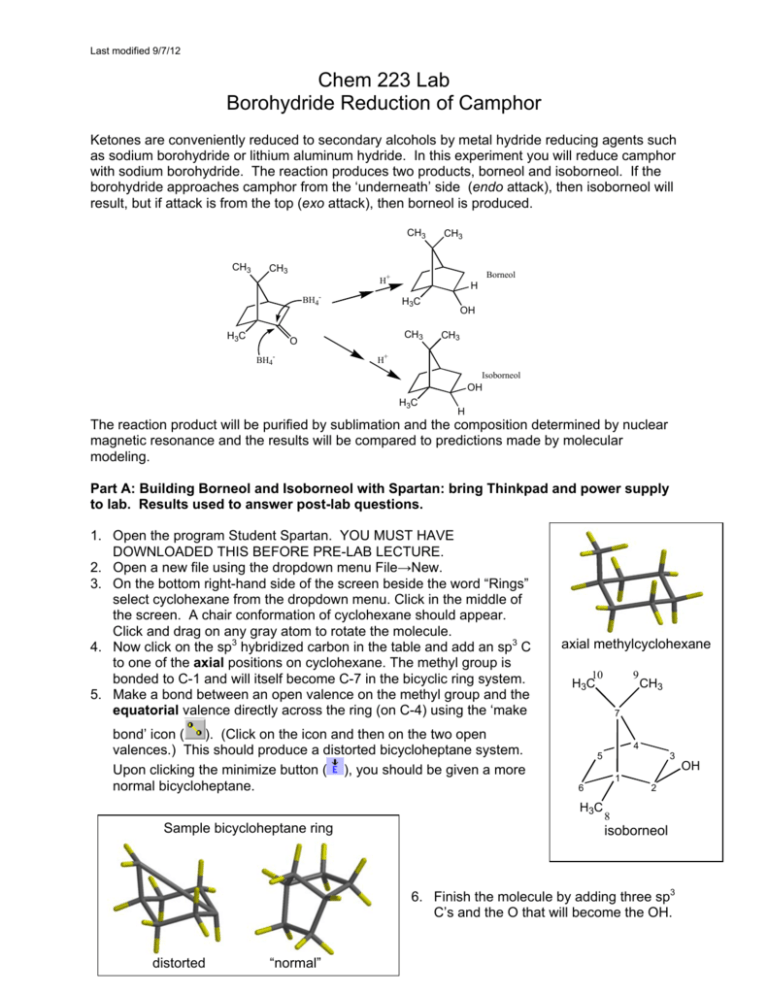
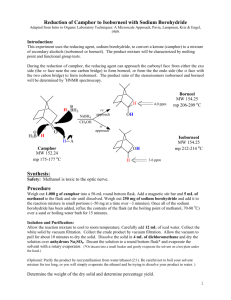
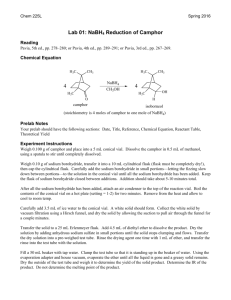
Last modified 9/7/12 Chem 223 Lab Borohydride Reduction of Camphor Ketones are conveniently reduced to secondary alcohols by metal hydride reducing agents such as sodium borohydride or lithium aluminum hydride. In this experiment you will reduce camphor with sodium borohydride. The reaction produces two products, borneol and isoborneol. If the borohydride approaches camphor from the ‘underneath’ side (endo attack), then isoborneol will result, but if attack is from the top (exo attack), then borneol is produced. CH3 CH3 CH3 Borneol H+ BH4- H3C CH3 H3C CH3 O BH4- H OH CH3 H+ Isoborneol OH H3C H The reaction product will be purified by sublimation and the composition determined by nuclear magnetic resonance and the results will be compared to predictions made by molecular modeling. Part A: Building Borneol and Isoborneol with Spartan: bring Thinkpad and power supply to lab. Results used to answer post-lab questions. 1. Open the program Student Spartan. YOU MUST HAVE DOWNLOADED THIS BEFORE PRE-LAB LECTURE. 2. Open a new file using the dropdown menu File→New. 3. On the bottom right-hand side of the screen beside the word “Rings” select cyclohexane from the dropdown menu. Click in the middle of the screen. A chair conformation of cyclohexane should appear. Click and drag on any gray atom to rotate the molecule. 4. Now click on the sp3 hybridized carbon in the table and add an sp3 C to one of the axial positions on cyclohexane. The methyl group is bonded to C-1 and will itself become C-7 in the bicyclic ring system. 5. Make a bond between an open valence on the methyl group and the equatorial valence directly across the ring (on C-4) using the ‘make bond’ icon ( ). (Click on the icon and then on the two open valences.) This should produce a distorted bicycloheptane system. Upon clicking the minimize button ( ), you should be given a more normal bicycloheptane. axial methylcyclohexane 10 CH3 7 4 5 1 6 H3C Sample bicycloheptane ring 9 H3C 3 2 8 isoborneol 6. Finish the molecule by adding three sp3 C’s and the O that will become the OH. distorted “normal” OH Last modified 9/7/12 (Add OH exo for isoborneol or endo for borneol.) Recall the Spartan will assume that any remaining open valences are bonds to H. 7. Click . 8. Click and carefully check the structure you have built. If you need to alter the structure, click to get back into the builder. 9. Save the file as either borneol or isoborneol, depending on which one you built. Finding the Lowest Energy Conformations of Borneol and Isoborneol: 10. You should have either borneol or isoborneol open. For clarity, close any other files. 11. Use the dropdown menu Setup Calculations. Choose "Equilibrium Conformer". Click submit. 12. Once the calculation is complete, use the dropdown menu Display→Output to view the results. Scroll down the display window until you see “Lowest Energy Conformation.” Write down the energy value, which is given in kJ/mole. 13. Run a new calculation to obtain the molecule’s heat of formation. Use the dropdown menu Setup Calculations. Choose "Equilibrium Geometry" with the “Semi-Empirical” PM3 model. Click submit. 14. Once the calculation is complete, use the dropdown menu Display→Output to view the results. Scroll down the display window until you see “Heat of Formation.” Write down the energy value, which is given in kJ/mole. 15. If you have not already done so, build the second reaction product. It is easy to convert borneol to isoborneol (and vice versa). First, change the file name to the name of the compound you will make. Then go to the builder ( ). Delete ( ) the oxygen. Choose the divalent oxygen in the chart and then add the O to the other position (endo if it was exo or exo if it was endo.) Minimize the energy ( ) and view the new structure ( ). 16. Repeat steps 10-14 for the other molecule. To View and Capture the Lowest Energy Conformation Images: 17. Close all Spartan files. 18. Open the file containing the minimized E conformer of either borneol or isoborneol. 19. Use the dropdown menu Model Tube to display the molecule in a less bulky format. 20. Position the molecule in the Spartan window EXACTLY as you wish it to appear in Word. 21. Under the dropdown menu File→Save Image As. Click OK and save the file on your laptop. Insert this file as an image into your lab report so your TA can see your lowest energy conformation of each molecule. Under Above the molecule, write its name, and below it give its heat of formation in kJ/mol. 22. Repeat steps 17-21 for the other reaction product. Part B: Reaction Procedure Place 2 mL of methanol in a 10 mL rb flask, and add 0.5 g of camphor. Swirl until the camphor dissolves, and then add 0.3 g of sodium borohydride. CAUTION. Do not add the borohydride all at once; divide it into four or five portions and add over a period of about five minutes, with swirling. Cool the flask in an ice bath, as necessary, to keep at room temperature. After the last portion of borohydride has been added, heat to gently boiling for one minute on a hotplate. Pour the hot reaction mixture into a 50 mL beaker that is about 2/3 full of crushed ice. Complete the transfer by rinsing the reaction flask with 2 mL of methanol. Swirl the beaker occasionally, and when the ice melts, collect the white solid by suction filtration and allow the material to air dry for about 30 minutesa. You could hasten the drying by blotting the crystals with a dry paper towel and gentle warming on a hotplate. Purify the material by sublimation. Transfer the crystals to a dry100 mL beaker. Place the beaker directly on a hot plate and make a ‘tent’ of Last modified 9/7/12 aluminum foil around the sides of the beaker and the base of the hotplate. In this manner, when heat is applied, the sides, as well as the bottom, of the beaker will be heated. Atop the beaker place a clean (on the outside!) 100 mL round-bottomed flask half-full of room-temperature water. The bottom of this flask should make contact with the top of the beaker. Apply heat. You should notice the appearance of product on the upper sides of the beaker and on the bottom of the flaska. Periodically check the temperature of the water in the flask; if it warms to above 40o, add crushed ice via a powder funnel such that the temperature drops below 30. Ideally, all the material would sublime onto the flask. However, it is likely that some of your product will remain on the side of the beaker. When no more white material remains in the bottom of the beaker discontinue heating and scrape the product off the bottom of the flaskb,c. Add to this product any crystals that you can scrape from the upper sides of the beaker. Determine your yield and turn in the product in a labeled vial to the instructor. At the end of the lab a representative sample will be taken to the NMR lab for analysis. a If the product is wet, water droplets will appear on the bottom of the round-bottomed flask. Do not despair; simply blot the surface dry and continue. b If you have too much product for sublimation, it may be necessary to momentarily interrupt the process to scrape sublimate off into a vial and then continue with the remainder. c Some inorganic boron-containing residue may remain in the bottom of the flask. Product Analysis (ON YOUR OWN TIME WITH MNova) View, integrate, and print the 1H NMRs of authentic samples of camphor, borneol and isoborneol locatedon the lab website. Examine the spectra to find places where they differ. Probably the best place to distinguish between borneol and isoborneol is between 3.4 and 4.2 , but you may find another that you like better. View the spectrum of the reaction product. Determine the composition of the product (camphor, borneol, and isoborneol) by analysis of the integrated spectrum. Report the composition of the product. Is there any unreacted camphor present? Include printed copies of your processed NMRs with your lab report, as well as a section called NMR analysis where you explain how you analyzed the product mixture. Postlab Questions 1. Find the lowest energy conformer of both borneol and isoborneol and its energy. Include a printout in your report that shows each lowest energy conformation and its energy in kcal/mole. a. Which compound is more stable, borneol or isoborneol? b. Can you suggest the likely source of the strain that is present in the less stable isomer? Hint: Use Spartan to look for crowding as evidenced by distorted bond angles. You’ll need to measure bond angles with Spartan. With a structure open, click and then 3 atoms. Spartan will display the angle in the bottom right corner of the screen. Be careful when selecting the atoms. The angle displayed is the angle about the 2nd atom you choose, so choose them in order! 2. Is the lower energy product the major product of the reaction based on NMR data? Is the reaction proceeding under thermodynamic or kinetic control? H CH3 3. If the borohydride reduction is performed on the compound shown to the right, the exo vs. endo percents are almost exactly reversed as compared to camphor. Suggest an explanation for this reversal in product stereochemistry. 4. Give a method other than 1H NMR that could be used to look for unreacted camphor in this experiment. H3C O