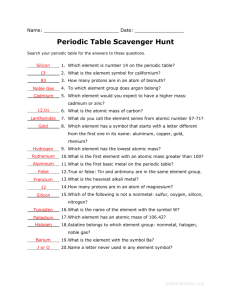
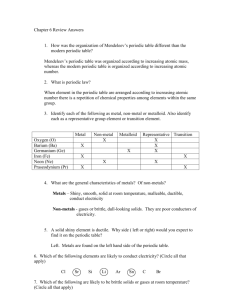
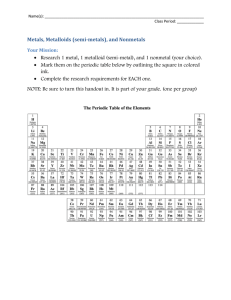
The state of matter of most of the elements to the left of the stair
advertisement

The state of matter of most of the elements to the left of the stair-step line in the periodic table is _____ . A. gas B. liquid C. plasma D. solid The state of matter of most of the elements to the left of the stair-step line in the periodic table is _____ . A. gas B. liquid C. plasma D. solid If a pattern repeats itself, it is _____ . A. isotopic B. metallic C. periodic D. transition If a pattern repeats itself, it is _____ . A. isotopic B. metallic C. periodic D. transition _____ is an element that would have similar properties to those of neon. A. Aluminum B. Argon C. Arsenic D. Silver _____ is an element that would have similar properties to those of neon. A. Aluminum B. Argon C. Arsenic D. Silver Boron is a _____ . A. metal B. metalloid C. noble gas D. nonmetal Boron is a _____ . A. metal B. metalloid C. noble gas D. nonmetal The element potassium is a _____ . A. metal B. metalloid C. nonmetal D. transition element The element potassium is a _____ . A. metal B. metalloid C. nonmetal D. transition element The element bromine is a _____ . A. metal B. metalloid C. nonmetal D. transition element The element bromine is a _____ . A. metal B. metalloid C. nonmetal D. transition element The halogens are those elements in Group _____ . A. 1 B. 11 C. 15 D. 17 The halogens are those elements in Group _____ . A. 1 B. 11 C. 15 D. 17 In its group, nitrogen is the only element that is a _____ . A. gas B. metalloid C. metal D. liquid In its group, nitrogen is the only element that is a _____ . A. gas B. metalloid C. metal D. liquid _____ is a shiny element that conducts electricity and heat well. A. Chlorine B. Sulfur C. Hydrogen D. Magnesium _____ is a shiny element that conducts electricity and heat well. A. Chlorine B. Sulfur C. Hydrogen D. Magnesium The atomic number of Re is 75. The atomic mass of one of its isotopes is 186. How many neutrons are in an atom of this isotope? A. 75 B. 111 C. 186 D. 261 The atomic number of Re is 75. The atomic mass of one of its isotopes is 186. How many neutrons are in an atom of this isotope? A. 75 B. 111 C. 186 D. 261 Negatively charged particles called ______ move around the nucleus. Negatively charged particles called neutrons move around the nucleus. Elements are arranged in the periodic table according to repeated changes in their ______ . Elements are arranged in the periodic table according to repeated changes in their properties . Vertical columns on the periodic table are called _____. Vertical columns on the periodic table are called groups . Hafnium-178 and hafnium-179 are examples of ______. Hafnium-178 and hafnium-179 are examples of isotopes . Cadmium’s atomic number is 48. The number of protons in a cadmium atom is ___ . Cadmium’s atomic number is 48. The number of protons in a cadmium atom is 48 .