Mystery Powders and Chemical Indicators
advertisement

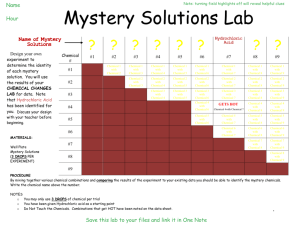
UC Irvine FOCUS! 5 E Lesson Plan Title: Mystery Powders and Chemical Indicators Grade Level and Course: 8th grade Physical Science Materials: 1. Spatula (recommend coffee stirrers or straw cut at an angle) 2. Eyedroppers or pipettes 3. Small disposable cups 4. Toothpicks for stirring 5. Testing solutions: dilute iodine solution, vinegar, water Note: iodine solution is readily available as a disinfectant at drug stores as a 10% solution. This can be further diluted several times and still work fine. 6. Mystery Powders: baking soda, corn starch, baking powder, powder sugar 7. Engage demonstration materials, including potato, bread, alka-seltzer tablet Instructional Resources Used: (concept maps, websites, think-pair-share, video clips, random selection of students etc.) Demonstration materials (listed below) Think-Pair-Share Internet resources 1. http://www.middleschoolscience.com/myspow.html 2. http://www.cedarville.edu/personal/lee/project/labs/lab-whitepowder.pdf 3. http://school.apostleslutheran.net/class/LabSkills/Mystery%20Powders%2 0Lab%20Activity.pdf 4. http://teacherweb.com/TX/BoerneChampionHighSchool/drmills/IPC_Ch05. 3_Mystery_Powder_Lab.pdf California State Standards: (written out) 8.5.a: Students know reactant atoms and molecules interact to form products with different chemical properties 8.7.c: Students know substances can be classified by their properties Common Core State Standards: (written out) Lesson Objectives: Students will use three “indicators” (water, vinegar, dilute iodine solution) to identify four unknown “mystery powders” Students will observe evidences of chemical reactions, such as color change (iodine-starch reaction) and production of gas (carbon dioxide bubbles) Differentiation Strategies to meet the needs of diverse learners: English Learners: EL students will be grouped with English proficient students for “Think-PairShare” and conducting the investigation Special Education: Depending on the number of special education students and the nature of this investigation, it may be recommended that this activity be done as a demonstration, perhaps using a document camera-projector for all to see GATE: Students explore other powders provided by the teacher. Examples include flour, rice powder, salt, talcum powder, cleanser, etc. ENGAGE Describe how the teacher will capture the students’ interest. Teacher will demonstrate the concept of “indicators”. For example, 1. Demonstrate iodine solution dropped on a piece of bread or potato. The iodine will react with the starch and turn a very dark purple/black color. The color change “indicates” the presence of starch. 2. Demonstrate an alka-seltzer tablet in water. The fizzing/bubbles “indicates” a chemical reaction may be occurring between the water and tablet. In this case, carbon dioxide is produced in the reaction. 3. Students participate in a think-pair-share to discuss evidences of a “chemical reaction.” a. possible color change c. temperature change b. production of gas (bubbles) d. precipitate forms (Note: c. and d. are not evident in this lab experience, so students may or may not come up with them on their own) What kind of questions should the students ask themselves after the engagement? 1. What is meant by an “indicator”? 2. What evidence may we observe that a “chemical reaction” may be occurring? EXPLORE Describe the hands-on laboratory activity that the students will be doing. 1. Students will follow all lab safety guidelines, including safety eye wear 2. Teacher will set out four “mystery powders” in labeled cups (A,B,C,D) 3. Teacher will set out three “indicators” liquids in labeled dropper bottles or in labeled cups with pipettes 4. Students will use spatula to place a small amount of powder “A” in three disposable cups. Then, using a pipette, a. add 60 drops of water to one cup and stir with toothpick b. add 20 drops of vinegar to second cup and stir with toothpick c. add 10 drops of iodine solution to third cup and stir with toothpick 5. Student should rinse/discard disposable cups & toothpicks 6. Students will record results of each indicator test for mystery powder “A” 7. Students repeat step 4-6 with the other three powders (B,C and D) List the “big idea” conceptual questions that the teacher will ask to focus the student exploration. 1. Why is it important to thoroughly rinse or discard your mixing sticks and cups after each part of the procedure? 2. Why is it important to carefully record your results after each test? 3. What is an “indicator” in a chemical test and how can an “indicator” help identify an unknown substance? 4. What are other examples of indicators in daily life (such as swimming pool test kits, soil test kits) EXPLAIN What is the “big idea” concept that students should have internalized from doing the exploration? Indicators are valuable tools scientists use to identify unknown substances. Indicators cause a chemical reaction which results in new substances being formed (eg: bubbles of carbon dioxide from vinegar+baking soda) List the higher order questions that the teacher will ask to solicit student explanations for their laboratory outcomes, and justify their explanations. 1. What are evidences that a chemical reaction may be occurring? 2. How is a scientist similar to a detective solving a crime? EXTEND Explain how students will develop a more sophisticated understanding of the concept. 1. Other “mystery powders” may be used (see links listed above; the internet has many very sophisticated “mystery powder labs available) 2. This activity can be used in a larger acid-base unit in which other indicators and other substances are identified. For example, using “cabbage juice” as an acid-base indicator. The cabbage “juice” turns “pink” in the presence of an acid, remains purple in a neutral solution, and turns “green” in the presence of a base. How is this knowledge applied in our daily lives? Indicators are used commonly among scientists, maintaining a swimming pool, or testing soils for planting. EVALUATE How will the student demonstrate their learning? Students will successfully identify the four unknown “mystery powders.” What is the learning product for the lesson? Students will gain experience with chemical indicators, identifying unknown materials, and 8th grade chemistry standards 8.5a and 8.7c. Background Knowledge for the Teacher: There are many common indicators used by scientists to determine unknown substances. There are dozens of acid-base indicators alone. People who manage a swimming pool use indicators to determine bacteria and chlorine levels. Indicators exist for testing what is needed in soils for specific plants. The color change in an indicator test is itself a chemical reaction with an equilibrium shift It is more common to refer to the vinegar as an “identifying reagent” when used as an indicator as in this activity. This “Mystery Powder” activity highlights similarities between a “scientist” and a “detective.” Both use “clues” to come to a “conclusion” and must be able to support their inferences with evidence. Following is a list of expected results in the indicator tests in this investigation Mystery Powder Baking Soda Baking Powder Corn Starch Sugar Reacts with water No Yes No No *Attach student pages to this lesson plan. Reacts with vinegar Yes Yes No No Turns Dark with iodine No No Yes No Name ___________________________________________________________ Period Science ____________ Science Detective Mystery Powder Lab Gather Evidence to Support Your Conclusion! This investigation has four “mystery powders” to identify. We will use three different “indicators” and record the results of our tests. Like a detective, look for clues and find the answer! Step One Step Two Step Three Scoop a small amount of mystery powder “A” into three small cups Add “indicator” solution to each cup then stir carefully with a toothpick Cup 1 = 60 drops of water Cup 2= 20 drops of vinegar Cup 3= 10 drops of iodine Look for evidence of a “chemical reaction” Record the results of your tests on the data table below Repeat this procedure with the other three powders. Use this chart to determine each powder! Powder Baking Powder Reacts with Water? Yes Reacts with Vinegar? Yes Reacts with Iodine? No Baking Soda No Yes No Corn Starch No No Yes Powder Sugar No No No Mystery Powder A B C D Add 60 drops water & mix Add 20 drops vinegar & mix Add 10 drops Iodine & mix Our Prediction? (We think this powder is … Identity of “Mystery Powders!” Were your predictions correct? Powder A was ________________________________________ Prediction correct? _______yes ______no Powder B was ________________________________________ Prediction correct? _______yes ______no Powder C was ________________________________________ Prediction correct? _______yes ______no Powder D was ________________________________________ Prediction correct? ______ yes ______no Short Answer Questions! 1. What is meant by a “chemical reaction”? _________________________________________________________ ___________________________________________________________________________________________________________ 2. What are some “evidences” that a chemical reaction may have occurred? ____________________ ___________________________________________________________________________________________________________ 3. List three common examples of a chemical reaction (for example, burning wood, rusting iron) a. first example ____________________________________________________________ b. second example _______________________________________________________ c. third example __________________________________________________________ 4. List three ways a scientist and a police detective are alike! For example, both are trying to solve a mystery! The scientist is trying to solve a mystery in nature and the police detective is trying to solve a crime! a. Scientists and police detectives both ________________________________________________________________ __________________________________________________________________________________________________________ b. Scientists and police detectives both ________________________________________________________________ __________________________________________________________________________________________________________ c. Scientists and police detectives both ________________________________________________________________ __________________________________________________________________________________________________________