O O H H H H O
advertisement

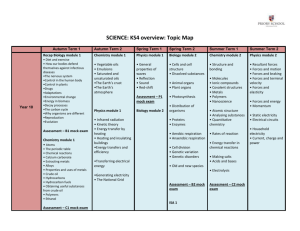
Mock Final Exam Chemistry 212 - Meyer Section I: Structure Exploration & Drawing Structures 1) In the box below each compound, list as many lipid categories as possibleto which the compound belongs. If a substance is NOT a lipid, write “not a lipid” in the box. 2) Circle all isoprene units in all terpenes shown in question 1. 3) Three out of the 4 structures drawn in question 1 are colored white. The other structure has a yellow color. Circle the structure that you expect to be colored yellow. Retinol is yellow. 4) Draw a wax derived from Lauric Acid and “Compound X” from question 1. O H O H O H 1 H Mock Final Exam Chemistry 212 - Meyer 5) When a certain triacylglycerol is hydrolyzed with sulfuric acid and water, the only products are glycerol and lauric acid. Draw this triacylglycerol O O O O O O 6) Draw a disaccharide that a β–1,4' glycosidic linkage between two D-glucopyranose carbohydrates. If there are any anomeric carbons that can spontaneously interconvert between two forms, put a star (*) next to each of these and draw them all in the α conformation. Make sure you draw this disaccharide using Haworth projections. HO HO O O HO HO O * HO OH OH OH 7) In the space below, draw a tripeptide at biological pH with the following amino acid sequence: Ala–Phe–Ala. The side chains are as follows: Ala = CH3; Phe = CH2Ph H3N H N O Ph O O N H O 2 Mock Final Exam Chemistry 212 - Meyer 8) In the space below, draw a phospholipid that would form one saturated fatty acid with twelve carbons and one unsaturated fatty acid with 14 carbons when it is hydrolyzed with acid and water. The phospholipid that you draw should contain a nitrogen atom that has bonds to three methyl groups. O O O O P O O O N O Section II: Predict the products and reagents 9. In the space provided, draw the correct structure of the major organic product(s). Indicate stereochemistry when appropriate. Also, indicate if a pair of enantiomers has been formed. 3 Mock Final Exam Chemistry 212 - Meyer H CN H H Δ CN OMe CN OMe OMe Br O HBr OH 10. Fill in the reactants necessary to convert iso-propyl ethyl ketone into the following substances: 4 Mock Final Exam Chemistry 212 - Meyer 11. Fill in the missing products in the following roadmap synthesis 5 Mock Final Exam Chemistry 212 - Meyer Section III: Mechanisms 12. Draw the complete mechanism and predict the major product(s) of the following reaction. O O O H3O+ O Δ tautomerize CO2 OH O H O H2O H O O O H O O H H+ transfer O O H O O HO H OH H2O O O O H H + EtOH OH 13. Draw the complete mechanism and predict the major product(s) for the following reaction. O Ru O Ru Ru (regenerated catalyst) O O O Ru Ru Ru H2C CH2 6 Mock Final Exam Chemistry 212 - Meyer Section IV: Retrosynthesis 14. Synthesize the following target molecule using acetylene as your only source of carbons. You may use as many equivalents of acetylene necessary. Other organic byproducts may be formed. H H O H O H H H O H H O O3 DMS H2/Lindlar 1. H2/Lindlar 2. HBr Br H Br2 Br Br H H2/Lindlar H HBr Br 7 Mock Final Exam Chemistry 212 - Meyer 15. Synthesize the following target molecule using acetone as your only source of carbons. You may use as many equivalents of acetone necessary. O OH O O O LAH OH O O NaOEt PBr3 Br Mg MgBr OH 8 Mock Final Exam Chemistry 212 - Meyer 16. Synthesize the following target molecule using dibromomethane and acetylene as your only source of carbons. You may use as many equivalents of acetylene necessary. Other organic byproducts may be formed. Br Br H H NaH Br Br H H 1.H2/Lindlar 2. a. BH3/THF b. H2O2/NaOH PBr3 Br (2 eq.) HO Br OH 1. H2/Lindlar 2. O3 / DMS 3. LAH Ru H2SO4 heat HO HO 9 Mock Final Exam Chemistry 212 - Meyer Section V: Spectroscopy 17. Answer the following questions about the structure given below. a. How many signals do you expect to see in the 13C NMR spectrum?____6____ b. How many signals do you expect to see in the 1H NMR spectrum?____4____ c. How many signals do you expect to see in the DEPT 135 spectrum?____4_____ 18. Deduce the structure that corresponds to the spectral data on the remaining pages of this exam. If the answer is incorrect, your analysis of the spectra can be worth significant partial credit, so show your work clearly in the space below each set of data only. Answers outside of these places will be ignored. Mass spectrum: m/z = 160 (M; 100%), m/z = 161 (9.0%), and m/z = 162 (0.9%). No fluorine or iodine. Molecular Formula: C8H16O3 DUN__________1_________ 10 Mock Final Exam Chemistry 212 - Meyer IR 1H-NMR: 4.93 ppm (septet; integral = 1), 3.73 ppm (triplet; integral = 2), 3.50 ppm (quartet; integral = 2),2.42 ppm (triplet; integral = 2), 1.32 ppm (doublet; integral = 6), and 1.10 ppm (triplet; integral = 3). Two Answers are acceptable: 11