Physics 41 Chapter 21 HW Set 1
advertisement
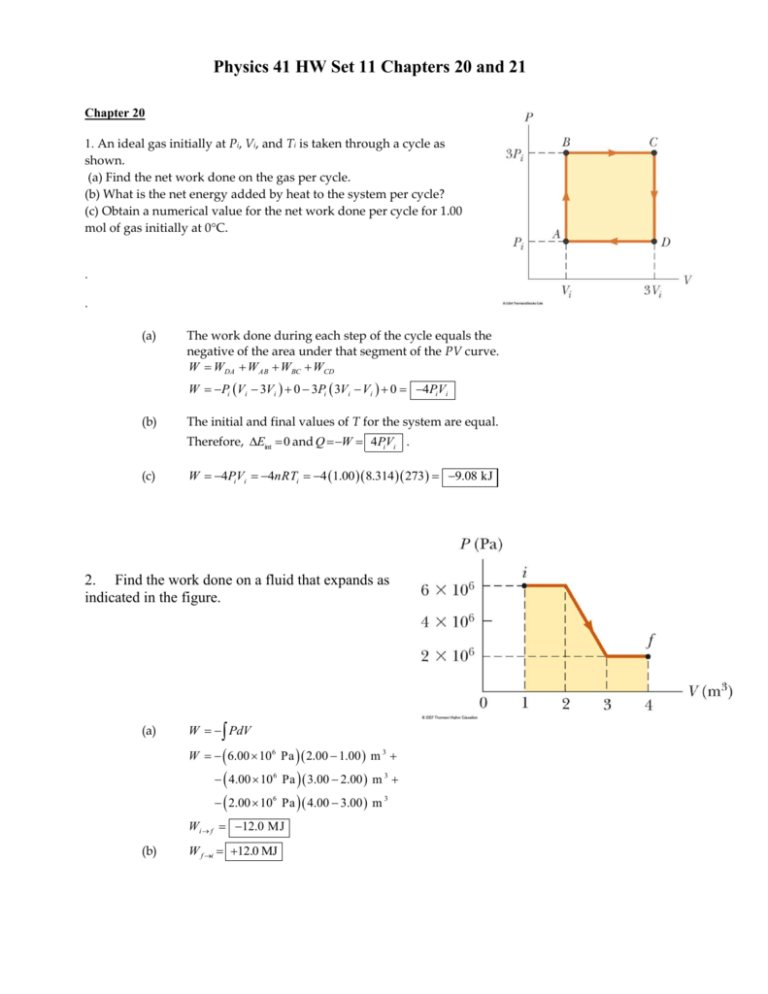
Physics 41 HW Set 11 Chapters 20 and 21 Chapter 20 1. An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown. (a) Find the net work done on the gas per cycle. (b) What is the net energy added by heat to the system per cycle? (c) Obtain a numerical value for the net work done per cycle for 1.00 mol of gas initially at 0°C. . . (a) The work done during each step of the cycle equals the negative of the area under that segment of the PV curve. W WDA W AB WBC WCD W Pi Vi 3Vi 0 3Pi 3Vi Vi 0 4PV i i (b) The initial and final values of T for the system are equal. Therefore, Eint 0 and Q W 4PiVi . (c) W 4PV i i 4nRTi 4 1.00 8.314 273 9.08 kJ 2. Find the work done on a fluid that expands as indicated in the figure. (a) W PdV W 6.00 106 Pa 2.00 1.00 m 3 4.00 106 Pa 3.00 2.00 m 3 2.00 10 6 Pa 4.00 3.00 m 3 Wi f 12.0 MJ (b) W f i 12.0 MJ 3. A sample of ideal gas is expanded to twice its original volume of 1.00 m3 in a quasi-static process for which P = V 2, with = 5.00 atm/m6, as shown. How much work is done on the expanding gas? f Wif PdV i The work done on the gas is the negative of the area under the curve 2 P V between Vi and V f . f 1 Wif V 2 dV V f3 Vi3 3 i V f 2Vi 2 1.00 m 3 2.00 m 3 3 3 1 Wif 5.00 atm m 6 1.013 10 5 Pa atm 2.00 m 3 1.00 m 3 1.18 MJ 3 4. An ideal gas is carried through a thermodynamic cycle consisting of two isobaric and two isothermal processes as shown. Show that the net work done on the gas in the entire cycle is given by W net P1 V2 V1 ln P2 P1 W W AB W BC W CD W D A B C D A C D W PdV PdV PdV PdV A B B W nRT1 A C D A dV dV P2 dV nRT2 P1 dV V V B C D V V W nRT1 ln B P2 VC V B nRT2 ln 2 P1 V A V D V1 VC Now P1V A P2VB and P2VC P1VD , so only the logarithmic terms do not cancel out. Also, VB P1 V P and 2 2 V1 P2 VC P1 W P P P P P nRT1 ln 1 nRT2 ln 2 nRT1 ln 2 nRT2 ln 2 nR T2 T1 ln 2 P2 P1 P1 P1 P1 Moreover P1V2 nRT2 and P1V1 nRT1 W P P1 V 2 V1 ln 2 P1 4. The inside of a hollow cylinder is maintained at a temperature Ta while the outside is at a lower temperature, Tb . The wall of the cylinder has a thermal conductivity k. Ignoring end effects, show that the rate of energy conduction from the inner to the outer surface in the radial direction is T Tb dQ 2Lk a dt ln b / a (Suggestions: The temperature gradient is dT/dr. Note that a radial energy current passes through a concentric cylinder of area 2rL.) For a cylindrical shell of radius r, height L, and thickness dr, the equation for thermal conduction, dQ dT becomes kA dt dx dQ dT k 2 rL dt dr Under equilibrium conditions, dQ is dt constant; therefore, dT Tb Ta dQ 1 dr dt 2 kL r dT dQ 1 b dr dt 2 kL a r Tb Ta But and dQ 1 b ln dt 2 kL a Ta Tb , so dQ 2 kL Ta Tb dt ln b a 5. In an insulated vessel, 250 g of ice at 0°C is added to 600 g of water at 18.0°C. (a) What is the final temperature of the system? (b) How much ice remains when the system reaches equilibrium? The latent heat of fusion is 3.33x105 J/kg and the specific heat of water is 4186 J/kg C°. (a) Since the heat required to melt 250 g of ice at 0°C exceeds the heat required to cool 600 g of water from 18°C to 0°C, the final temperature of the system (water + ice) must be 0C . (b) Let m represent the mass of ice that melts before the system reaches equilibrium at 0°C. Qcold Qhot mL f m w cw 0C Ti m 3.33 10 5 J kg 0.600 kg 4 186 J kg C 0C 18.0C m 136 g, so the ice rem aining 250 g 136 g 114 g 6. An ideal gas initially at 300 K undergoes an isobaric expansion at 2.50 kPa. If the volume increases from 1.00 m3 to 3.00 m3 and 12.5 kJ is transferred to the gas by heat, what are (a) the change in its internal energy and (b) its final temperature? (a) Eint Q PV 12.5 kJ 2.50 kPa 3.00 1.00 m 3 7.50 kJ (b) V1 V 2 T1 T2 V 3.00 T2 2 T1 300 K 900 K V1 1.00 Chapter 21 7. A cylinder contains a mixture of helium and argon gas in equilibrium at 150°C. (a) What is the average kinetic energy for each type of gas molecule? (b) What is the root-mean-square speed of each type of molecule? (a) K (b) 3 3 kBT 1.38 1023 J K 423 K 8.76 1021 J 2 2 K 1 2 m vrm s 8.76 1021 J 2 vrm s so 1.75 1020 J m For helium, m 4.00 g m ol 6.02 1023 m olecules m ol 6.64 1024 g m olecule m 6.64 1027 kg m olecule Similarly for argon, m 39.9 g m ol 6.02 1023 m olecules m ol 6.63 1023 g m olecule m 6.63 1026 kg m olecule Substituting in (1) above, we find for helium, vrm s 1.62 km s and for argon, vrm s 514 m s (1) 8. A 1.00-mol sample of hydrogen gas is heated at constant pressure from 300 K to 420 K. Calculate (a) the energy transferred to the gas by heat, (b) the increase in its internal energy, and (c) the work done on the gas. We us the tabulated values for C P and C V (a) Q nC P T 1.00 m ol 28.8 J m ol K 420 300 K 3.46 kJ (b) Eint nCV T 1.00 m ol 20.4 J m ol K 120 K 2.45 kJ (c) W Q Eint 3.46 kJ 2.45 kJ 1.01 kJ 9. A 2.00-mol sample of a diatomic ideal gas expands slowly and adiabatically from a pressure of 5.00 atm and a volume of 12.0 L to a final volume of 30.0 L. (a) What is the final pressure of the gas? (b) What are the initial and final temperatures? (c) Find Q, W, and Eint. (a) PV i i PfV f 1.40 V 12.0 Pf Pi i 5.00 atm 1.39 atm 30.0 Vf (b) Ti Tf (c) 5.00 1.013 105 Pa 12.0 103 m PV i i nR 2.00 m ol 8.314 J m ol K PfV f nR 3 1.39 1.013 105 Pa 30.0 103 m 2.00 m ol 8.314 J m ol K 3 365 K 253 K The process is adiabatic: Q 0 1.40 C P R CV 5 , CV R CV CV 2 5 Eint nCV T 2.00 m ol 8.314 J m ol K 253 K 365 K 4.66 kJ 2 W Eint Q 4.66 kJ 0 4.66 kJ 10. Fifteen identical particles have various speeds: one has a speed of 2.00 m/s; two have speeds of 3.00 m/s; three have speeds of 5.00 m/s; four have speeds of 7.00 m/s; three have speeds of 9.00 m/s; and two have speeds of 12.0 m/s. Find (a) the average speed, (b) the rms speed, and (c) the most probable speed of these particles. (a) vav (b) nivi N v 2 av 1 1 2 2 3 3 5 4 7 3 9 212 6.80 m s 15 nivi2 54.9 m 2 N so vrm s (c) v 2 s2 54.9 7.41 m s av vm p 7.00 m s 11. In an ultrahigh vacuum system, the pressure is measured to be 1.00 10–10 torr (where 1 torr = 133 Pa). Assuming the molecular diameter is 3.00 10–10 m, the average molecular speed is 500 m/s, and the temperature is 300 K, find (a) the number of molecules in a volume of 1.00 m 3, (b) the mean free path of the molecules, and (c) the collision frequency. (a) N PV N A so that PV RT and N RT N A 1.00 10 1331.00 6.02 10 10 N (b) 23 8.314 300 1 nV d2 21 2 V N d2 21 2 779 km (c) f v 6.42 104 s1 3.21 10 12 3.21 1012 m olecules 1.00 m 3 m olecules 3.00 1010 m 2 21 2