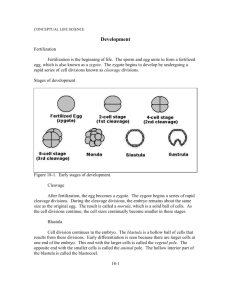
Summary of the main patterns of cleavage
advertisement

Model organisms in development Cell and embryology A few have been studied extensively; each has advantages and disadvantages. Model Systems Xenopus laevis: development is independent (in vitro), easy catch and observation but poor genetics. Model organisms: vertebrates (frog, mouse, zebrafish) Model organisms: invertebrates (sea urchin, Drosophila, nematode) Chick: available, surgical manipulation and in vitro culture but poor genetics. Identifying development genes Mouse: surgical manipulation, good genetics, transgenic model, mammalian but development p is in utero . Textbook: Wolpert L, Beddington R, Jessell T, Lawrence P, Meyerowitz E, Smith J. (2007) Principles of Development. 3th ed. London: Oxford university press. Drosophila: great genetics, great development (recent Nobel Prize to Lewis, Nusslein-Volhard & Wiechaus). C. elegans: has less than 1000 cells and is transparent. Gilbert SF. (2003) Development Biology. 7th ed. Sunderland: Sinaure Associates Inc. Sea Urchin : in vitro 1 Arabidopsis thaliana: flowering plant. 2 Summary of the main patterns of cleavage Lecithal 3 4 1 Model organisms: vertebrates All vertebrate embryos undergo a similar pattern of development. 1) fertilization 2) Cleavage (cell number ↑, but total mass X) Fi 2 1 Fig.2.1 3) blastulation (blastcoel formation and three germ layers) The skeleton of a mouse embryo illustrates the vertebrate body plan 4) gastrulation (where ectoderm covers embryo, endoderm and mesoderm are inside), A-P axis (body plan), notochord formation, embryo affected by yolk in egg. In mammalian, yolk to small but have extra-embryonic structure of placenta for nutrition nutrition. 5) Phylotypic stage, at which they all more or less resemble each other an show the specific features of notochord, somites and neural tube. Fig. 2.2 5 The phylotypic stage Xenopus laevis: egg (Amphibians) At the end of gastrulation all embryos appear to be similar (the phylotypic stage). Structures that are common to the phylotypic stage of the vertebrates are: 1) the notochord (an early mesoderm structure along A/P axis), 2) the somites (blocks of mesoderm on either side of notochord which form the muscles of the trunk & limbs), 3) the neural tube - ectoderm above notochord forms a tube (brain and spinal cord). Vertebrate embryo to through a phylotypic state, but differences in form before gastrulation 6 Advantage: easy observation, fertilized, catch (sperm, egg), low infection Extraembryo nic tissue The egg is composed of an animal and a vegetal region, i b both th covered db by vitelline it lli membrane b ((gell coat). Fig.2.4 Meiosis is stopped at 1st division with apparent 1 polar body (the 2nd polar body comes after fertilization). Box 2A After fertilization, the cortex (the layer below plasma membrane) rotates to determine future dorsal region at a position opposite to the site of sperm entry. entry Animal vegetal Fig. 2.3 7 8 2 Box 2A Cleavage of a frog egg. 9 10 Xenopus laevis : fertilization and early growth Early developmental stages of Xenopus laevis 1. one sperm enters animal region (grow to embryo, plant pore to yolk) morula Blastula 2. completes meiosis 3. egg and sperm nuclei fuse 4 vitelline 4. it lli membrane b lift lifts 5. yolk rotates down (15 minutes) 6. cortical rotation occurs (60 minutes). 囊胚 7. 1st cleavage occurs (90 mins) Animal / Vegetal (A/V) 8. Every 20 mins, one cleavage 2.5 hpf p 3.5 hpf p 5 hpf p 9. 2nd cleavage (110 mins) A/V 90 degrees to 1st 10 hpf p 10. 3rd cleavage (130 mins) equatorial (4 small animal and 4 large vegetal= 8 , it is blastomeres). blastocoel - 11. Continued cleavage → blastomeres ↓, cells at vegetal region large than those at the animal region. hpf: hours post-fertilization 11 12 3 Xenopus laevis: blastulation The blastula (after 12 divisions) has radial symmetry. The marginal zone will become mesoderm and endoderm. Marginal zone, the belt of tissue around the equator , plays a crucial part in future development. Internalization of the mesoderm and endoderm starts at the blastopore. Fig 2.3 Life cycle of the frog Xenopus laevis. In blastula stage, it is in the form of a hollow sphere with radial symmetry 13 14 Xenopus laevis: gastrulation Types of cell movement during gastrulation Invagination Involution Ingression Delamination Eiboly: ectoderm covers embryo 15 Gastrulation step: 1. Mesoderm and endoderm converge and begin to move inwards at dorsal lip of the blastopore. 2. Mesoderm and endoderm extend in along A/P axis. 3. Ectoderm spreads to cover embryo (epiboly). 4. Dorsal endoderm separates mesoderm from the space between the yolk cells, the archenteron (future gut). Do not forget, mesoderm come from ectoderm 5 5. Lateral mesoderm spread to cover inside of archenteron archenteron. 6. dorsal mesoderm is beneath dorsal ectoderm 7. mesoderm spread to cover gut 8. epiboly - ectoderm covers embryo 9. yolk cells are internalized (food source), dorsal mesoderm develops into a) notochord (rod along dorsal midline) and b) somites (segmented blocks of mesoderm along notochord). Blastopore ↓ Archenteron ↓ Large ↓ Blastocoel ↓ Close ↓ gut 16 4 Xenopus laevis: Neurulation • Neuralation or neural tube formation: 1) The neural plate is the ectoderm located above notochord and somites. 2) The edge of the neural plate forms neural folds which rise towards midline. 3) The folds fuse to form neural tube. 4) The neural tube sinks below epidermis. • The anterior neural tube becomes brain. Mid and posterior neural tube becomes spinal cord. Gastrulation → neurulation → neural plate → fold → tube notochord Neural crest cell Anterior posterior ↓ Autonomic nerves ↓ 18 Brain spinal cord 17 Fig. 2.7 Neurulation in amphibian Xenopus laevis: Somites The somites formation, after neurulation The dorsal part of somites have ready begun to differentiate into dermatome (future dermis). The rest of each somite becomes vertebrae and trunk muscles (and limbs). Lateral plate mesoderm becomes heart, kidney, gonads and gut muscles. V Ventral l mesoderm d b becomes bl blood-forming df i tissues. i Also at this stage, the endoderm gives rise to the lining of the gut, liver & lungs. Brain and spinal Notochord begins to form in the midline Neural plate develops neural folds Fig. 2.8 A cross-section through a stage 22 Xenopus embryo just after gastrulation and neurlation are completed 19 20 5 The major lineages of the mesoderm Xenopus laevis: tail bud stage • After gastrulation comes the early tail bud stage In the anterior embryo: a) the brain is divided, b) eyes and ears form, c) 3 branchial arches form (anterior arch later becomes the jaw. In the posterior embryo, the tail is formed last from dorsal lip of blastopore by extension of notochord, somites and neural tube. Circulatory body cavity system Scler Myo tome Cartilage skeletal Fig. 2 Fi 2.9 9 Th The early l ttailbud ilb d stage of Xenopus embryo dermis 21 22 Schematic representation of neural crest formation (in chick embryo) Xenopus laevis : neural crest cells Neural folds meet and adhere Neural crest cells come from the edges of the neural folds after neural tube fusion. Neural crest cells can form from the dorsal side of the closed neural tube Cells at this junction form neural crest Neural crest cells detach and migrate as single cells between the mesodermal tissues to become: 1) sensory and autonomic nervous systems 2) skull 3) pigment cells Closure not simultaneous 4) Cartilage → bone Only vertebrate Cell adhesion molecular expressed dependent Epidermal and neural plate/tube interactions may generate crest cells 23 Closed tube detaches – change in adhesion molecule expression 24 6 Zebrafish (Danio rerio rerio)) -- A Vertebrate Model •It is 3 cm long •Short generation time •Large clutch size •External fertilization •Transparent embryos •Rapid development http://zfin.org/ and http://www.nih.gov/science/models/zebrafish/ 25 26 Sphere 29h 48h 27 28 7 •Human disease model •Reverse genetics tool Fish (Zebrafish) embryo: •Transgenics Fig. 2.26 29 The development of Zebrafish 30 Characterization of Fish embryo Telolecithal: most of the egg cell is occupied by yolk Meroblastic: the cell divisions not completely divide the egg Discoidal: since only the blastodisc becomes the embryo, this type of meroblastic cleavage is call discoidal discoidal. Zebrafish development occurs very rapidly rapidly. In 24 hr hours of embryogenesis, shown here, the 1 cell zygote becomes into a vertebrate embryo with a tadpole-like form. Cleavage can take place only in the blastodisc, a thin region of yolk free cytoplasm at the animal pole of the egg. Fig. 2.27 Cleavage of the zebrafish embryo 31 32 8 Fish embryo: blastula stage Three cell populations: At about the 10th cell division -- the onset of the MBT mid-blastula transition 1. Yolk syncytial layer (YSL) 2. Deep cells -- forming the embryos proper 3 Envelope 3. E l layers l (EVL) -- forming f i the th epidermal id l ANIMAL BODY About 10 cell division, the onset of mid-blastula transition: gene transcription begins, divisions slow and cell move. And formed three distinct cell populations: (1)YSL (yolk syncytial layer): location of vegetal edge of the blastoderm and fusion produces a ring of nuclei within the part of the yolk cell cytoplasm that just beneath the blastoderm blastoderm. It is important for directing some of the cell movement of gastrulation. Internal YSL: the yolk syncytial nuclei move under the blastoderm External YSL: some cell move vegetally, stay ahead of the blastoderm margin (2)Enveloping layer (EVL): Made up of the most superficial cell from the blastoderm, which form an epithelial sheet a single cell layer thick. (3) Deep cells Blastoderm 4 hpf: hours post-fertilization 33 Both YSL and EVL are the deep cells, that give rise to the embryo proper. 34 Fish embryo: gastrulation The fate map of the deep cells after mixing has stopped Internal YSL The blastoderm at 30% completion of epiboly (4.8 hr) This stage, no mesoderm, ectoderm The fate of the early blastoderm cells are not determined. After much cell mixing during cleavage 35 36 9 Types of cell movement during gastrulation Formation of the hypoblast, either by involution of cells at the margin g of the epibolizing p g balstoderm or by delamination and ingression of cells from the epiblast (6hr) The formation of germ layers is started. Close-up of the marginal region Invagination Involution Ingression Delamination Eiboly: ectoderm covers embryo 37 About 90% epiboly (9 hr), mesoderm can be seen surrounding the yolk, between the endoderm and ectoderm 38 Types of cell movement during gastrulation Complete gastrulation (10.3hr) Invagination I Involution l ti Ingression Delamination Eiboly: ectoderm covers embryo 39 40 10 Fish embryo: gastrulation Fig 2.28 Epiboly and gastrulation in the zebrafish Convergence and extension in the gastrula. After fertilization → cell cleavage → spreading out of the layer of cell (epiboly) → upper half of the yolk become covered by a cup-shaped blastoderm→ gastrulation by involution of cell → fromed a ring around the edge of the blastoderm → involuting cell converge on the dorsal midline to form the body of the embryo 41 Mesodermal cell ( (expressed d snailil gene)) flank the notochord (A) Dorsal view of convergence and externsion movements during gastrulation. Epiboly spreads the blastoderm over the yolk; involution or ingression generates the hypoblast; convergence and extension bring the hypoblast and epiblast cells to the dorsal side to form the embryonic shield. (B) Convergent extension of the embryo; it is show by cells expression the gene no tail 42 (a gene is expressed by notochord cells) Types of cell movement during gastrulation Invagination Involution Ingression Delamination Eiboly: ectoderm covers embryo 43 44 11 Chick (bird) embryo: the blastodisc (blastoderm) Chicken The blastodisc arises through cleavage (20 hrs.). The blastodisc can be divided into two areas: 1) the area pellucida (a light area) surrounded by 2) the area opaca (a dark ring). 犁溝 yolk 45 Fig. 2.10 46 The life cycle of the chicken (Fig.2.11) 47 48 12 Discoidal meroblastic cleavage in a chick egg Chick (bird) embryo: the blastodisc (blastoderm) The posterior marginal zone forms at the junction of the area pellucida and the area opaca and defines the dorsal side and posterior end of the embryo. The hypoblast (the source of extraembryonic tissues) develops as a layer on top of yolk and develops from cells from the posterior marginal layer and the overlying cells of the blastoderm. It come from two sources: the posterior marginal zone, which lies at the junction between the opaca and pellucida at the posterior of the embryo. It develop to extraembryonic structure and related with epiblast. Fig. 2.12 Germinal opaca pellucida opaca ectoderm endoderm 49 Primitive streak Formation of two-layered blastoderm of the chick embryo 50 Types of cell movement during gastrulation Germinal (A,B) Primary hypoblast cells delaminate individually to form islands of cell beneath the epiblast (C) Secondary hypoblast cells from posterior margin → migrate beneath the epiblast and incorporated the polyinvagination islands → move anterior; As the hypoblast moves anteriorly → epiblast cell collect at the region anterior to Koller’s sickle to form the primitive streak Invagination Involution Ingression Delamination Eiboly: ectoderm covers embryo 51 52 13 Chick embryo: the primitive streak Chick embryo: the primitive streak The primitive streak is a slit or line on the disc which lays down the A/P axis. (posterior) Onset of gastrulation This structure begins to form from the posterior marginal zone and extends to a point in the central region of the disc disc. Cells move towards the streak, and mesoderm and endoderm internalize at this site. When the primitive streak reaches its greatest length (forward), the anterior end begins to regress back to the posterior end. Primitive streak form at posterior → forward formation → enough length close and regress → Hensen’s Hensen s node → backward regression → formation of head, somites and notochord… (Fig. 2.14) The anterior end of the regressing streak is known as Hensen's Node. Unlike amplibians, cell not only proliferation but also growth in size during size, gastrulation in bird and mammals. Primitive streak 53 54 The major lineages of the mesoderm Cell movement of the primitive streak of the chick embryo Head, somite Circulatory body cavity system Scler Myo tome 55 Cartilage skeletal dermis 56 14 Chick embryo: gastrulation As Hensen's Node moves toward the posterior, several structures form behind it: 1) The head fold (from ectoderm and endoderm) 2) The notochord and somites (from mesoderm) 3) The neural tube forms above the notochord (from ectoderm) (The anterior structures are formed first while the posterior structures are completed last.) 4) Neural folds fuse at the dorsal midline and neural crest cells migrate away 5) The head fold separate, gut forms and heart pieces fuse to form heart. 57 58 Fig.2.18 Development of the chick embryo Chick embryo: neurulation notochord Neural plate → neural fold → meet midline Intermediate mesoderm→ kidney Splanchnic mesoderm → heat somites Somite star formation 13 somites Hensen’s node 59 20 somites 40 somites 60 15 Mouse embryo Chick embryo: extra-embryonic structure Amnion and amniotic cavity provide mechanical protection Chorion maintain shell Allantois bridge for oxygen and waste Vitelline vein take nutrient form yolk to embryo Umbilical vein take oxygen to embryo Fig.2.20 Egg is small, 100mm very small Egg surrounded by protective external coat, zona pellucida 61 62 Development of a human embryo form fertilization to implantation Mouse embryo: fertilization Fertilization occurs in oviduct. (Fig. 11.26) Cleavage occurs in oviduct: 1st at 24 hours and every 12 hours after that to form the morula (a ball of cells). (Fig. 2.21) • Blastomere compaction happens at 8 cell stage. • Smooth inner membranes and outer membranes are covered with microvilli. (b) Four-cell stage. Remnants of the mitotic spindle can be seen between the two cells that have just completed the second cleavage division. (c) Morula. After further cleavage divisions, the embryo is a multicellular ball that is still surrounded by the fertilization envelope. The blastocoel cavity has begun to form. 63 64 16 Mouse embryo: In 16 cell morula → • Cleavage partitions the cytoplasm of one large cell – Into many smaller cells called blastomeres At ~16 cell morula, has two group cells. A small group of internal cell mass (ICM) surrounded by a large group of external (trophectoderm) cells. Trophectoderm: becomes extra-embryonic tissues (such as placenta). Inner cell mass (ICM): becomes the embryo plus some extraembryonic tissues. The morula (~32 cell stage) has 2 cell fates: 1) inner 8 cells (Inner Cell Mass) 2) outer ~20 cells (trophectoderm). blastocyst (a) Fertilized egg. Shown here is the (b) Four-cell stage. Remnants of the (c) Morula. After further cleavage divisions, the embryo is a zygote shortly before the first mitotic spindle can be seen multicellular ball that is still cleavage division, surrounded between the two cells that have surrounded by the fertilization by the fertilization envelope. just completed the second envelope. The blastocoel cavity The nucleus is visible in the cleavage division. has begun to form. center. (d) Blastula. A single layer of cells surrounds a large blastocoel cavity. Although not visible here, the fertilization envelope is still present; the embryo will soon hatch from it and begin swimming. 65 66 Mouse embryo: post-implantation Mouse embryo: blastocyst In the blastocyst (~3½ days), the trophectoderm and ICM are established. Fluid is pumped in to expand cavity and increase the size of the blastocyst. blastocyst: preimplantation (3½ - 4½ days) The surface of ICM will become the primitive endoderm while the remaining becomes primitive ectoderm (= ( epiblast). epiblast) Implantation occurs. The zona pellucida is discarded and blastocyst attaches to uterine wall. Uterine wall hypoblast Development of a human embryo form fertilization to implantation Implantation → trophoblast giant cell invade → trophoectoderm grows → ectoplacental cone & extra-embryonic ectoderm → primitive endoderm cover inner surface of trophectoderm → to visceral endoderm • 67 In the first two days post-implantation, the mural trophectoderm (cells that are not in contact with the ECM) gives rise to polyploid trophoblast giant cells. • The rest of trophectoderm becomes the ectoplacental cone and the extra-embryonic ectoderm which give rise to the placenta. • Primitive mesoderm migrates: 1) to cover inner surface of mural trophectoderm to become the parietal (腔壁) endoderm and 2) to cover egg cylinder and epiblast to become the viseral endoderm • Six days after fertilization, the epiblast is cup-shaped. 68 17 Mouse embryo: gastrulation 6½ days after fertilization: The primitive streak forms at the start of gastrulation at the future posterior end. (Inside cup is future dorsal side) Cells move through the streak and spread forward and laterally between the ectoderm and the visceral endoderm to form the mesoderm. Later the definitive endoderm (from epiblast) will replace the visceral Later, endoderm. The primitive steak first elongates, then at the anterior tip of the primitive streak, the node forms. (The node formed from anterior → posterior) Then notochord and somites form anterior to the node (A/P axis). Cells migrate through mesoderm to form endoderm (gut). Epiblast move through the primitive streak to give rise to the mesoderm and definitive endoderm. 69 Amnion Chorion Allantois Fig. 2.23 Mouse embryo: late embryogenesis (neurulation) Mouse embryo: final stages of gastrulation • By 8½ days after fertilization, 1) the neural folds form at anterior and dorsal, and 2) the embryonic endoderm internalizes to form the gut. • 9 days after fertilization embryogenesis is complete. 1. 2. 3. 4 4. 5. 6. Fig. 2.24 A 70 Complex folding Initially on the ventral surface of embryo Internalize to form the gut Heat and liver move into their positions Head becomes distinct Embryo surrounded by extra-embryonic membrane P D Primitive streak extend→ produce extra-embryonic structure →chorion, amino, allantois The primitive streak similar to chick (node = Hensen’s node) Organogenesis in the anterior part Neural folds formation Amnion Chorion Allantois Fig. 2.25 71 72 18 Diagram showing the timing of human monozygotic twinning with relation to extra-embryonic membrane Formation of the notochord in the mouse Amnion Chorion Allantois 73 74 Model organism: invertebrate Drosophila melanogaster: early embryogenesis The Drosophila egg is the shape of a sausage . Meroblastic (superficial) cleavage and centrolecithal It has a micropyle at the anterior end (site of sperm entry). With fertilization, the fusion of nuclei is followed by rapid mitotic divisions (9 minutes) and no cytoplasmic cleavage. A syncytium is formed (many nuclei/common cytoplasm). After nine divisions, nuclei move to the periphery to form the syncytial blastoderm . Fig. 2.29 Life cycle of Drosophila Fig. 2.30 75 After fertilization, no cell was form, but rapid nuclear division in a cytoplasm 76 19 Box 2A Drosophila: embryogenesis By 13 mitoses the membranes sprout to surround the nuclei to form cells (cellular blastoderm). ~15 cells at posterior (= pole cells) are sequestered and become the germline. g During first ~3 hrs large molecules such as proteins can move between nuclei until the cellularization occurs. Single layer of cells give rise to all tissues (syncytium ). Gastrulation starts at ~3 hrs. Mesoderm forms from ventral tissue, midgut from endoderm at the anterior and posterior ends ends, ectoderm remains on outside outside. During gastrulation, the ventral blastoderm (germ band), comprises extension. The mesodermal tube forms from ventral tissue then cells separate and move to internal locations under the ectoderm. 77 78 Drosophila melanogaster: gastrulation The mesoderm becomes muscle and connective tissues. In insects, nerve cord lies ventrally (vertebrates: dorsal). Neuroblasts form a layer between mesoderm and outer ectoderm. midgut (anterior & posterior) grow from threads and fuse. = anterior and posterior midgut ectoderm becomes epidermis. No cell division occurs during gastrulation. Afterward, division restarts. Future mesoderm invaginate ventral region → intrnalized tube → cell leave tube and migrate under the ectoderm The surface of ventral blastoderm → cell leave and form a layer between ventral ectoderm and mesoderm Anterior and posterior invaginate and fuse → gut Midgut →region endoderm Foregut and hindgut → ectodermal origin 79 80 20 Future mesoderm invaginate ventral region → internalized tube → cell leave tube and migrate under the ectoderm Ventral view Fig. 2.31 Gastrulation Dorsal view germline The surface of ventral blastoderm → cell leave and form a layer between ventral ectoderm and mesoderm →nervous system Anterior and posterior invaginate and fuse → gut Midgut →region endoderm Foregut and hindgut → ectodermal origin 81 82 Drosophila melanogaster: segmentation Drosophila melanogaster: larvae The germ band (ventral blastoderm) is main trunk region. Germ band extension pushes posterior end over dorsal side. The first signs of segmentation grooves appear to outline parasegments (early embryo) which give rise to segments (late embryo). Segments are formed from the posterior of one parasegment and the anterior of the next. (formed form posterior to anterior) The larvae hatch at 24 hrs post-fertilization. Larval structures of note include: The anterior end is the acron. The posterior end is the telson. Along with the head, the larvae has 3 thoractic segments and 8 abdominal segments. The ventral side of the larvae has denticle belts, alternating patches of denticle hairs and cuticle on each segment, segment used for locomotion. Fig. 2.32 There are 14 parasegments: Fig. 2.33 3 mouth, 3 thorax, 8 abdominal. 83 84 21 Drosophila melanogaster: metamorphosis Three instar stages of larval life are separated by molts. • 1st instar 2nd instar 3rd instar molt molt 3rd instar larvae forms pupae (pupa) to undergo metamorphosis. The adult tissues arise from imaginal discs and histoblasts. imaginal discs: small sheets of epidermis (~40 cells each of cellular blastoderm) which grow throughout larval life. Imaginal discs: 6 leg, 2 wing, 2 haltere, 2 eye-antenna, plus genital, head discs and ~10 histoblasts: nest of cells in the abdomen which give rise to the abdominal segments. imaginal discs histoblasts Larval epidermis degeneration begins prior to imaginal disc eversion Imaginal disc cells and histoblasts will replace the larval epidermis Formation of adult abdominal segments - gene expression in histoblasts Imaginal discs Fig. 2.34 Imaginal discs vs. adult structure Antenna haltere Genitalia 85 86 Caenorhabditis elegans: the model of nematode THE WORM After gastrulation In case of self-fertilization there are ~ 0.1 - 0.3% male worms in the population. Fig. 2.35 Life cycle of nematode http://www.wormatlas.org/handbook/contents.htm 87 88 22 Press Release: The 2002 Nobel Prize in Physiology or Medicine 7 October 2002 The Nobel Assembly at Karolinska Institutet has today decided to award The Nobel Prize in Physiology or Medicine for 2002 jointly to the model of nematode Small nematodes that are 1 mm long and 70 µm in diameter. 19,000 gene Small number of cell (558, first larval stage) T Transparency off embryo, b and d growth th rapid id The adult hermaphrodite (maless can develop) undergo rapid development. The egg has a 50 µm diameter which forms a polar body after fertilization, nuclear fusion occurs followed by a set pattern of cleavage. The normal pattern of cell division has been mapped. Many cells undergo programmed cell death. Hermaphrodite: 959 cells from 1090 somatic nuclei of which 131 undergo programmed cell death; 300 germ cells undergo apoptosis; 116 of the 131 dying cells are cells of the nervous system and ectoderm Sydney Brenner, H. Robert Horvitz and John E. Sulston for their discoveries concerning "genetic regulation of organ development and programmed cell death" 1927 89 1947 1942 90 Molecular Regulation of Apoptosis C. elegans mutagenize Non- apoptotic apoptotic wildtype CED mutants (Cell Death abnormality) 91 92 23 Fig. 2.36 Cleavage of the nematode embryo Fertilization →polar bodies formation → asymmetric cleavage → anterior AB cell, smaller posterior P1 cell DIC image Fig. 2.38 elegans larva at the L1 stage. Fig.2.37 Cell lineage and cell fate in the early nematode embryo Anus Pharynx Primordium 93 Sea Urchin: blastula formation Invertebrate: Sea Urchin Radial holoblastic cleavage (isolecithal) The 4th cleavage, very different from the first three. In animal pole, four cell divide to 8 blastomeres and with the same volume (the 8 cells also called mesomeres). In vegetal pole, undergoes an unequal cleavage to four large cells (macromeres) and four small cells (micromeres). The animal mesomeres divide equatorially to produced two tiers: an1 and an2. The vegetal macromeres divide a small cluster beneath the large tier. (not equal) 128 cells blastula. 94 The blastula stage of sea urchin development begins at the 128 cells. Blastulation: The cells form a hollow sphere surrounding a central cavity (blastocoel). Every cell contact with proteinaceous fluid of the bastoceol (inside) and with the hyaline layer on the outside. About 9th or 10th cleavage, cells become specified and they end develop cilia. Ciliated blastula → rotate within fertilization envelop (E→F) → vegetal pole of Bastula become thicken (forming vegetal plate) → then animal pole synthesis and secret hatching enzyme → digest fertilization envelope → embryo is a free swimming hatched blastula. 4th cleavage Meridionally rotate 95 96 24 Fate maps and the determination of sea urchin blastomeres Fate map of the zygote Late blastula with ciliary tuft and flattened vegetal plate blastula Fate map and cell lineage of the sea urchin. 97 98 Formation of syncytial cables by primary mesenchyme cells of sea urchin SEM of spicules formed by the fusing of primary mesenchyme cells into syncytial y y cables Prism-stage larva Gastrulation star C: SEM of primary mesenchyme cells enmeshed in the extracellular matrix of early y gastrula. g D: Gastrula-stage mesenchyme cell migration Pluteus larva The extracellular matrix fibrils of the bastocoel lie parallel to the animal-vegetal axix 99 100 25 Ingression of primary mesenchyme cells Invagination of the vegetal plate SEM of external surface off the th early l gastrula t l CSPG release → into inner lamina → osmotic gradient ↑→ absorb water → swell inner lamina ,but outer lamina attached does not swell → inward Fertilization envelope 101 CSPG: chondroitin sulfate proteoglycan 102 Identification of developmentally important genes Entire sequence of gastrulation in sea urchin The developmental genetics of Drosophila and mice are best known. Homologous genes identified in these organisms are found in other species. Dominant (or semi-dominant) mutations: one copy of mutant gene produces mutant state. These are more easily recoginzed, they don’t cause the eayly death of the embryo in the heterozygous. Recessive mutations: two copies of a mutant gene gives the mutant state. Allele: The gene is contributed by the male and female Homozygous: both alleles of a pair carry the mutation Heterozygous: just one copy of the mutant gene is present 103 104 26 Recessive mutation vs. Semi-dominant mutation -/- Most mutations are recessive, but usually die in embryo. 105 106 Developmental gene can be identified by induced mutation and screening Genetic screening to produced homozygous yg mutant zerbrafish embryo Heterozygous Embryos homozygou s the induced mutation will be found in the offspring of 25% of the matings heterozygous 107 108 27 Mutagenesis and genetic screening strategy for identifying developmental mutants in Dorsophila main patterns of cleavage phylotypic stage DTS: dominant temperaturesensitive mutation, up 29oC → death b: a non non-developmental developmental lethal recessive Time vs. developmental events T Types off cell ll movementt during d i gastrulation t l ti Primitive streak gastrulation Neurulation ethyl methane sulfonate human monozygotic twinning Syncytium imaginal discs and histoblasts 109 Dominant (or semi-dominant) mutations 110 28