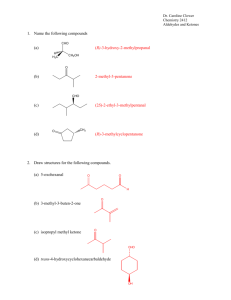
Chapter16 Conjugate Addition
advertisement

Chapter 16 Conjugate Addition Conjugate Pi bonds Hydrocarbon containing Different Kinds of Dienes two double bonds: diene three double bonds: triene four double bonds: tetraene many double bonds: polyene Carbonyls compounds with conjugated Pi bond: A Molecular Orbital Description of Stability Stability of dienes Why is conjugated diene more stable than isolated diene diene? ? H2C H2C CH CH CH CH CH2 CH2 H2C CH CH CH2 resonance contributors CH2 CH CH CH2 resonance hybrid The Molecular Orbitals of 1,3 1,3--Butadiene Bonding MO: constructive (in-phase) overlap Antibonding MO: destructive (out-of-phase) overlap HOMO--LUMO HOMO The highest-energy molecular orbital of 1,3-butadiene that contains electrons is ψ2 (HOMO) The lowest-energy molecular orbital of 1,3-butadiene that does not contain electrons is ψ3 (LUMO) HOMO = the highest occupied molecular orbital LUMO = the lowest unoccupied orbital HOMO--LUMO HOMO π*2 π*1 E π2 E π*2 π*4 π1 π3 1,2--Addition & 1,4 1,2 1,4--Addition to conjugated dienes π1 For a conjugated diene For an isolated diene Electrophilic Addition Reactions of Isolated Dienes Electrophilic Addition Reactions of conjugated Dienes When only one mole of HBr is added, two products will be formed Addition occurs as if we have 2 separate double bonds If only one mole of HBr is added, one of the double bonds The product resulting from the reaction on the adjacent will undergo addition sp2 carbons is called the 1 1,2 1,22-addition product The product resulting from the reaction on the conjugated carbons is called the 1,41,4-addition product Mechanism of Electrophilic Addition Kinetic versus Thermodynamic control Kinetic control Thermodynamic control Most stable Kinetic versus Thermodynamic control Kinetic versus Thermodynamic control The thermodynamic product is the most stable product The thermodynamic product predominates when the reaction is reversible (thermodynamic control) The kinetic product is the product that is formed most rapidly The kinetic product predominates when the reaction is irreversible (kinetic control) The 1,41,4-addition product has the greater number of alkyl groups bonded to the sp2 carbon Æ MORE STABLE 1,4--Addition Polymers 1,4 The Diels Diels--Alder Reaction unstretched polymer stretched rubber Conformations of Dienes H H H H H H Conformations of Dienes H s-trans H H H H HH s-cis s prefix designates conformation around single bond regardless of the stereochemistry at each double bond s prefix is lower case (different from CahnCahn-IngoldIngold-Prelog case) S which designates configuration and is upper case) H H H H H H s-trans H H H HH s-cis s prefix designates conformation around single bond s prefix is lower case (different from CahnCahn-IngoldIngoldPrelog S which designates configuration and is upper case) Conformations of Dienes s-trans The Diels Diels– –Alder Reaction Reaction:: a 1,41,4-Addition Reaction s-cis Both conformations allow electron delocalization via overlap of p orbitals to give extended π system The Diels Diels– –Alder Reaction: some examples The conformation of the diene must be ss--cis to have Diels--Alder reaction Diels The conformation of the diene must be ss--cis to have Diels--Alder reaction Diels The Diels Diels– –Alder Reaction: some examples Two Possible Configurations of Bridged Bicyclic Compounds Effects of Conjugation in α,β--Unsaturated Aldehydes and α,β Ketones R and CH2 are trans R and CH2 are Cis Resonance Description Acrolein O H2C •• – O •• •• CHCH O •• C C C C C C + •• •• – O •• C +C C •• Properties Properties α,β-Unsaturated aldehydes and ketones are α,βmore polar than simple aldehydes and ketones. α,β-Unsaturated aldehydes and ketones are α,βmore polar than simple aldehydes and ketones. α,β-Unsaturated aldehydes and ketones contain α,βtwo possible sites for nucleophiles to attack α,β-Unsaturated aldehydes and ketones contain α,βtwo possible sites for nucleophiles to attack carbonyl carbon carbonyl carbon β-carbon β-carbon •• O •• C βC C Dipole Moments O δ– δ+ δ+ O δ– δ+ Conjugate Addition to µ = 2.7 D µ = 3.7 D α,β--Unsaturated Carbonyl Compounds α,β Butanal trans--2-Butenal trans greater separation of positive and negative charge Nucleophilic Addition to α,β-Unsaturated Aldehydes and Ketones 1,2--addition (direct addition) 1,2 Kinetic versus Thermodynamic Control attack is faster at C=O nucleophile attacks carbon of C=O attack at β-carbon gives the more stable product 1,4--addition (conjugate addition) 1,4 nucleophile attacks β-carbon O O C C C C + H Y C C 1,2--addition 1,2 H O C C C Y + H Y 1,4--addition 1,4 formed faster major product under conditions of kinetic control (i.e. when addition is not readily reversible) enoll goes to keto form under reaction conditions H O C Y C C O O C C C C + H C Y 1,4--addition 1,4 k t fform is keto i iisolated l t d product of 1,41,4-addition is more stable than 1,2--addition product 1,2 H 1,2--addition 1,2 1,4--addition 1,4 O C C + H C=O is stronger O Y C C 1,2--Addition 1,2 C O than C=C C H Y C Y Y C C C Example O observed with strongly basic nucleophiles CH3CH CHCH + HC Grignard reagents g CMgBr 1. THF 2. H3O+ LiAlH4 NaBH4 OH Sodium acetylide strongly basic nucleophiles add irreversibly CH3CH CHCHC (84%) CH H Example 1,4--Addition 1,4 O observed with weakly basic nucleophiles C6H5CH CHCC6H5 cyanide ion (CN–) thiolate ions ethanol, KCN (RS–) acetic acid ammonia and amines azide ion (N3 O –) C6H5CHCH2CC6H5 weakly basic nucleophiles add reversibly CN (93--96%) (93 Example Example O C6H5CH KCN CHCC6H5 ethanol, •• via O •• C6H5CH – CH •• CC6H5 CN CH3 acetic acid CN (93--96%) (93 HO–, H2O C6H5CH2SH •• – •• O •• O C6H5CHCH2CC6H5 O C6H5CH CH O CC6H5 CH3 CN (58%) SCH2C6H5 Example •• O •• via O •• – CH3 SCH2C6H5 CH3 α,β--Unsaturated Carbonyl Compounds α,β •• – •• O •• O CH3 (58%) Conjugate Addition of Organocopper Reagents to HO–, H2O C6H5CH2SH SCH2C6H5 CH3 SCH2C6H5 Addition of Organocopper Reagents to α,β-Unsaturated Aldehydes and Ketones Example O + LiCu( LiCu(CH CH3)2 CH3 The main use of organocopper reagents is to form carbon carbon--carbon bonds by conjugate addition to α,β-unsaturated ketones. 1. diethyl ether 2. H2O O CH3 CH3 (98%)