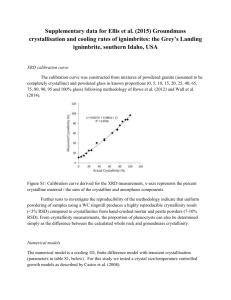
Mapping crystallization space to see where to go: the C6 Web Tool
advertisement

THE C6 WEBTOOL Mapping crystallization space to see where to go Janet Newman Uppsala, May 2011 COLLABORATIVE CRYSTALLISATION CENTRE (C3) • History: • • • • • • Grant application in 2004 (Bio21 NPCF) First equipment in 2005 (Fluidigm Topaz system) CSIRO first equipment in 2006 First plates 9-03-2006 On-line booking July 2006 CSIRO C3 July 2010 • Numbers • • • • Users – 158 Blocks created – 855 Crystal plates – 10239 (distinct reservoir_designs in xtal plates) – 1055 CSIRO. Core Facilities WHAT IS A SCREEN? •A screen is a collection of crystallants •There are often 24, 48 or 96 conditions in a screen •The crystallant •“reservoir” “well” “conditions” “cocktail” “precipitant” “precipitating solution” •consists of 2-5 different chemicals, from a set of 300-500 chemicals •buffer (0.1M) •salt (0.2-2M) •polymer (5-30%) •Screens •Available commercially •Made locally QUESTIONS THAT ARE OFTEN ASKED OF US: •What screen(s) should I use? • Which ones do you have in-house? •What screens do you have which contain/don‟t contain chemical x? •I‟ve set up screen Y, what should I try now? •Literature conditions for protein Z are available… IS THIS A PROBLEM? •Currently there are at least 230 commercially available screens • (195 in September, 2008) •≈14000 conditions WHICH SCREENS/CONDITIONS TO USE? •Use them all: •Assume setups of 100 nl protein +100 nl crystallant •~145 x 96 well plates need to be set up • over 3 ml protein • Around €30K • 5-10K for plastic consumables etc • 7 days to set up (in C3) • 200000 images to review CAN WE STREAMLINE THIS? • We could set up only the unique crystallants. • Still a big problem • And how do you work out which ones are unique? • Set up only the distinct crystallants QUESTIONS ABOUT „DISTINCTNESS‟ •Can we define “likeness” for crystallisation conditions? •When are two crystallants similar? • How different are they? •When are two crystallisation screens similar? • How different are they? DO THIS EXPERIMENTALLY (“OUTPUT”) Take a number of proteins Set up in each (unique) condition Observe… Conditions that produce the same crystals are similar PROBLEMS •How many proteins? •Which proteins? •What about other conditions? •How do we extrapolate to similarity between different screens? AND this assumes: If a crystal can happen, it will happen. Or to put it another way That crystallisation is a reliable process. REPLICATION EXPERIMENT •Lysozyme (same solution) •Hampton HT (4 different blocks-same batch) •Same pipetting robot (Phoenix) •Same imagers (Rigaku) •Same plate type (Greiner low profile) •Same seals/phase of moon/blah blah blah • • • • 50+50nl or 100+100nl drops 4C or 20C Imaged four days after setup Crystals detected by inspection of images NOT JUST LYSOZYME • Catalase • Set up 6 times in the HWI 1536 well screen • <85% correlation between the replicants AN “INPUT” WAY OF DETERMINING SIMILARITY •Look at the make-up of the crystallisation condition and compare that to the make-up of other crystallisation conditions What groundwork need to be in place? •Consider two conditions: • 100mM citric acid pH5 • 25% PEG MME 5K • 200mM MgSO4•7H2O • 0.1M sodium citrate pH 5.0 • 0.2M magnesium sulfate • 25w/v MPEG 5000 •Data standards • • • • Naming Spelling Units Ordering MEASURING DIVERSITY •A metric is a distance between two points in a space. •In normal Euclidian space, distance is given by •What about chemical space? METRICS •We define an initial dissimilarity metric for crystallisation space • T is the number of distinct chemical species in conditions i and j • [sti] is the concentration of chemical t in condition i • max[st] is the maximum concentration found for that chemical within chemical space (solubility) CRYSTALLISATION DISSIMILARITY METRIC •This returns a value between 0 and 1, where •0 means the two conditions are identical •1 means the two conditions are maximally dissimilar (nothing in common) Worked examples •Condition i • 100mM Hepes pH 7.5 • 200mM sodium acetate • 2M Ammonium sulfate •Condition j • 100mM MES pH 5.5 • 0.5M Ammonium sulfate • 20w/v PEG 4K 1/5 (1 + 1 + 1 + 1 + (2M-0.5M)/3.5M) = 0.88 • 100mM Acetate pH 4.6 • 200mM Li2SO4 • 20w/v PEG 3350 • 100mM Acetate pH 4.6 • 200mM MgCl2 • 25w/v PEG 3350 1/4 (0 + 1 + 1 + (25-20)/35) = 0.53 REFINEMENT OF THE INITIAL METRIC • Include a pH term, which uses information that is available about pH (to normalise this value, use a denominator which spans the pH range found over all commercial conditions 2.4-11.6) • Include a similarity term (based on name) into the metric, so that lithium sulfate will be more similar to lithium chloride than it will be to sodium acetate. (include a penalty for guessing) • Look at conditions which both have a single PEG species, and if 1/2 [PEGi]/[PEGj] 2 then term becomes ([PEGi]-[PEGj])/25 (includes the denominator, which is essentially a penalty for guessing) CURRENT METRIC + + COMPARING SCREENS •Two screens (or sets of conditions) may be compared, given a metric using •Not so good for comparing a set of conditions to itself • average distance better in this case DO WE HAVE TO GET THE METRIC “RIGHT”? How do you measure “rightness”? • The metric correctly finds all similar conditions for every protein? • The metric predicts as well as any crystallographer? OR • The metric enables one to answer questions that couldn‟t be answered before? THE C6 WEBTOOL • http://c6.csiro.au WHICH SCREENS ARE SIMILAR? •How similar are they? HOW SIMILAR ARE THEY? • Easier to see graphically CSIRO. Insert presentation title, do not remove CSIRO from start of footer HOW SIMILAR ARE THEY? • Similarity comes in different ways CSIRO. Insert presentation title, do not remove CSIRO from start of footer THERE ARE DIFFERENT TYPES OF SCREENS What type of screen is it? 1 0.9 0.8 Screen diversity 0.7 0.6 Minimum distance 0.5 Average distance 0.4 0.3 0.2 0.1 0 1 17 33 49 65 81 97 113 129 145 161 177 193 209 225 screen number SPY0210 conditions? SPY0210 conditions? What is in that screen? WHAT SHOULD REALLY BE IN THE METRIC? • Physico-chemical properties • The protein doesn‟t „see‟ ammonium sulfate • What might be important? • Specific activity • Dielectric constant • Viscosity • Surface tension • pH • conductivity •Acknowledgments •State Government of Victoria •Vincent Fazio •CSIRO bosses (Tom, Tim, Tim, Paul) •Shane, Tam, Christine •CSIRO IT •C3 Users Thank you Web: www.csiro.au/c3 Email: c3@csiro.au Twitter: @C3CSIRO PH ASSAY - BACKGROUND • The pH of the crystallisation experiment affects the charges of ionisable sidechains (asp, glu, his, arg, lys) • 30% of the commercially available crystallisation conditions are provided with no associated pH information. • The pH value is usually the pH of a minor “buffer” component • Currently there are 10 (ok, 9) vendors of crystallisation screens, 231 screens available, or 13,868 individual conditions • In c3 • 855 total deep well blocks • (475 are unique) • Any assay will be repeated A LOT. Should be cheap, fast and easy MEASURING PH • Litmus used since 1200s • extracted from one of several lichens – often Roccella tinctoria • Robert Boyle (1727-91) showed that the indicator could be adsorbed onto white paper • 1871 • phenolphthalein first synthesized – introduced in 1877 as an acid/base indicator (clear→fuchsia > pH 8.2) • 1935 • Beckman (California) and Radiometer (Denmark) produced electric pH meters (sensitivity 0.02 pH units) UNIVERSAL INDICATORS • Use combinations of different indicators to span a large pH range PH MEASUREMENTS IN HIGH-THROUGHPUT • Needs to be plate based • Needs to use small volumes of reagents • Accuracy not as important as precision • Yamada Universal Indicator (Bromothymol blue, thymol blue, Methyl red, Phenolphthalein) HOW BEST TO DO THIS? • Add 1:10 diluted dye to unknown solutions (dye:solution:water) • 50:50:0 • 50:25:25 • 50:10:40 • pH is concentration independent! • Dilution of solutions removes problem with intrinsic colour. HOW TO QUANTIFY THE COLOUR? • Create a standard curve with pH from 4 to 10 • (Use a plate reader with • Monochromator (not filters) • Measure from 300nm to 800nm) CHC curve (1:5 dye) 1 0.9 0.8 Absorbance 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 Wavelength WHY DOESN‟T THIS WORK? • Colours are a continuum – • different colours are formed by changes in relative absorbance over a wide spectrum of wavelengths, rather than by summing discrete peaks • cf. gel filtration curves – colours aren‟t well separated • Metamerism • two light sources, made up of different mixtures of various wavelengths, may appear to be the same colour • Colour detectors • Our colour detector (eye) not being uniformly sensitive to light of different wavelengths RGB COLOUR MATCH • Take an image of the dyed solution • Compare that to a standard curve using an interpolation of the Red Green Blue (RGB) hue values • Colours can be collected on the crystal imager • The images are passed through a program (pH Hue-ristic) to convert to pH values, and can be uploaded directed into our database HOW WELL DOES IT WORK? QUESTIONS • What chemicals should be used to make the standard curve? • What concentration should the dye be? • Does the camera that is used to take the pictures matter? • What could be done to get around the nonlinearity at around pH 7? Different cameras (temperatures) dye dilutions at 2 temps 600 500 400 Hue 1:10 1:20 300 1:10b 1:20b 200 100 0 3 3.5 4 4.5 5 5.5 6 6.5 7 pH 7.5 8 8.5 9 9.5 10 10.5 11 Comparison to pH meter values pH (pH meter) vs. pH (heuristic) 12 y = 0.9312x + 0.538 R² = 0.9309 pH measured with Hue-ristic 10 8 6 4 2 0 0 2 4 6 pH measured with pH meter 8 10 12 Comparison of the same screen over time Old New 3 2.5 2 1.5 1 0.5 0 -4 16 36 56 76 96 PEGs over time pH estimation for very small samples THE PERFECT WORLD OF MACROMOLECULAR CRYSTALLISATION • 100% accuracy with crystallisability prediction from sequence • “Rational” crystallisation: understand link between the crystallisation condition and the resultant packing in a crystal • Tools to get all salient information from an drop image • Crystal quality – tools for optimising diffraction from a crystal rather than visual perfection of the crystal