Spring 2015 CH421 Independent Project 3
advertisement

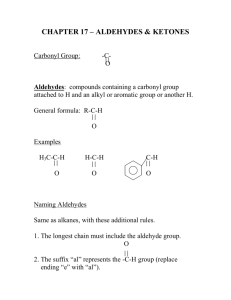
Spring 2015 CH421 Independent Project 3 The Cannizzaro Reaction: An Examination of Aldehyde Substituent Effects and Reaction Conditions Number of students in project group: 4 Project Overview For the final four weeks of CH421 laboratory, students will be split into a total of four groups, and each group will work on one of four different independent projects. For Independent Project 3, the main goal is to determine whether or not the nature of the substituent on the aldehyde (i.e. EDG or EWG) affects its ability to participate in the Cannizzaro reaction. This will be explored using two different types of reaction conditions (traditional reflux conditions vs. room temperature with a catalyst). The Cannizzaro reaction is a somewhat unusual reaction for a few reasons. First, hydroxide acts as a nucleophile and attacks the aldehyde, and we haven’t seen nucleophile/electrophile reactions of hydroxide (or alkoxide) nucleophiles with aldehyde or ketone electrophiles. Second, via a unique mechanism, redox chemistry occurs between two aldehydes, whereby one aldehyde molecule is reduced to the benzyl alcohol, while the other is oxidized to the carboxylic acid (see Scheme 1 below). Scheme 1: The Cannizzaro Reaction The group will perform two different Cannizzaro reactions under two different reaction conditions (four reactions total), whereby the para substituent of the aldehyde is varied for each reaction, as shown in Scheme 1. For each reaction, two products are generated, which must somehow be separated and purified. Each purified compound will then be characterized by IR and 1H NMR. In theory, the electronic nature of the substituent R group may influence the reactivity of the aldehyde in the Cannizzaro reaction. In order to determine whether or not there is a substituent effect, two aldehydes with different electronic properties (Cl and OMe) will be reacted. In addition, two different reaction conditions will be explored: the traditional conditions at reflux and room temperature conditions using a catalyst. Project Components and Point Distribution 1. 2. 3. 4. SciFinder literature search (20 points) Lab work – cleanliness and acumen (20 points) PowerPoint presentation of results (80 points) Final lab write‐up (80 points) 1. SciFinder Literature Search (20 points): due with final lab report on Wednesday April 29 by 5:00 pm Each pair of group members will conduct a search on SciFinder to find literature procedures for the preparation of the EXACT COMPOUNDS shown in Scheme 1. NOTE: only look for journal articles (not patents!) and note how many total journal articles that you found. Within each search answer set, specifically look for a journal article that reports conditions for room temperature Cannizzaro reactions. Make sure to show your search results to your instructor before proceeding! Save the PDF file for the room temperature conditions article to your H drive and email a copy to your instructor. Look through the procedure and note reaction conditions (i.e. base, solvent) and scale. Your findings should be typed up using an appropriate word processing program (i.e. MS Word) and emailed to your instructor. Be sure to include your lab section, project number, and “SciFinder search” in the file name. Spring 2015 CH421 Independent Project 3 2. Lab Work (cleanliness and acumen worth 20 points) NOTE: all laboratory work should be recorded in your laboratory notebook. A group of four (4) students will share the lab work to synthesize, purify, and characterize (IR, and 1H NMR) each of the alcohol and carboxylic acid products. The chemistry portion will be completed in the first three weeks of lab. NOTE: there is no procedure to follow here! You will perform the reactions starting with 1.0 g of the aldehyde, and you should decide the appropriate amounts of the base, what solvent(s) to use, appropriate glassware, how to separate your final products, etc. You may ask your instructor for guidance. For IR and NMR data of your starting materials, the following website is very helpful: http://sdbs.db.aist.go.jp/sdbs/cgi‐bin/cre_index.cgi. Suggested division of lab and SciFinder work: Week 1 o SciFinder Search (all group members – 1 hour) o FIRST, two pairs will set up the ROOM TEMPERATURE Cannizzaro reactions (one pair for each substituent: Cl and OMe). You will find that the reaction will take much more than 24 hours, so these reactions will be set up, stirred for 10 mins, and then set aside on a cork ring in the back of the hood for a week. Follow the progress of this reaction by TLC after 2 hours, and again the following week. o Next, set up the reactions under standard conditions (4 equiv. of 11M aq. KOH; reaction solvent is MeOH; reflux) for R = Cl and OMe. Monitor this reaction by TLC for disappearance of the starting aldehyde (the reaction should take more than 20 mins but less than 90 mins at reflux). Once the reaction is complete, work up this reaction. NOTE: you need to think about how to isolate these two products from each other using techniques that you’ve already learned! (HINT: you will not need to use column chromatography). Once you have a plan, check with your instructor before proceeding. o IF THERE IS TIME (you will need ~45 mins), isolate the alcohol and carboxylic acid products from each other and set aside in your hood for characterization in Week 2. Otherwise, wait until Week 2 for the workup. Week 2 o If you did not work up the standard conditions reaction from Week 1, do so here. o Characterize (IR and 1H NMR) the two products (aldehyde and carboxylic acid) from the standard conditions. o Perform a TLC of the room temperature Cannizzaro reactions to determine whether or not they are complete (they should be). Monitor this reaction by TLC for disappearance of the starting aldehyde. Then, work these reactions up in the same way that you worked up the standard conditions reactions. o IF THERE IS TIME, characterize (IR and 1H NMR) the two products (aldehyde and carboxylic acid) from the room temperature reactions. Otherwise, wait until Week 3 for characterization. Week 3 o Finish any remaining lab work, product characterization, record data in your notebook, and clean‐up. o Type up the SciFinder results. o Start work on your PowerPoint Presentation o Start typing up procedures for the final lab report. Week 4: PowerPoint presentation to be given in the lab. See details in Section 3 below. Spring 2015 CH421 Independent Project 3 3. PowerPoint Presentation of Results During the Final Lab Period (80 points): due Tues 4/21 or Thurs 4/23 Each project group will present their results to the class using PowerPoint (or some equivalent program). Each group member should give a portion of the presentation so that it is split up more or less evenly. Each slide should have an appropriate title. The PowerPoint presentation should include a slide for each of the following: Title slide with all group members listed Statement of the scientific purpose of the project Brief explanation of the Cannizzaro reaction mechanism (NOTE: it’s probably easiest to go through the mechanism by drawing it on the white erase board using 4‐chlorobenzaldehyde and KOH instead of trying to draw it on a drawing program. Leave this mechanism on the board for use again below). A slide showing the reaction scheme for the standard condition Cannizzaro reactions that were performed (i.e. just show “R” on the reaction scheme and label R = Cl and OMe), including the reaction conditions used. NOTE: each hallway computer has the drawing program ChemDraw installed, which is the appropriate software with which to draw your structures and paste into PowerPoint and into your final write‐up. As such, you should be sure to save your ChemDraw files to your H drive. A slide showing the reaction scheme for the room temperature Cannizzaro reactions that were performed (i.e. just show “R” on the reaction scheme and label R = Cl and OMe). Note how these reaction conditions differed from the standard conditions. Using the mechanism already drawn on the board, also describe how the catalyst and base that you used work in this reaction mechanism. A slide showing the results of your chemistry in table format, including columns for each of the following: conditions (standard or room tempterature), the R group, yield, purity of each final product and how that was determined (i.e. TLC, NMR, etc.), and a comment column to note differences (if any) in purity, yield or ease of use between the different conditions you tried. Conclusions slide with bullet point conclusions. The final PowerPoint presentation file should be emailed to your instructor for calculation of this part of your project grade. Be sure to include lab section and project number in the file name. Spring 2015 CH421 Independent Project 3 4. Final Lab Write‐Up (80 points): due on Wednesday April 29 by 5:00 pm Instead of grading individual lab notebooks (all of which should be kept perfectly at this point in the semester!), part of the independent project grade will come from a formal lab report (format discussed below). The report should be typed up using an appropriate word processing program (i.e. MS Word) and emailed to your instructor when complete. Be sure to include your lab section, project number, and “Lab Report” in the file name. The work should be divided evenly and compiled and edited together. The final report should be organized into several sections, in a similar same way that a chemistry journal article is organized. For an example, go to the Journal of Organic Chemistry website from a Regis computer, open a recent article, and refer to this for organization and style. The final report should be organized as follows: Title: Project name and number. Include names of all group members below title. Abstract: this is a succinct paragraph that starts with a general statement about the purpose of the experiment, and then proceeds to briefly describe the chemistry and methods used, and the results of the project. Introduction: this should have relatively brief paragraphs discussing the Cannizzaro reaction, its biochemistry equivalent reaction (see section 19.12 on biological reductions in McMurry), the different reaction conditions that you used (be sure to cite the journal article from which you obtained the room temperature procedure). Results and Discussion: first, discuss the general success of the chemistry and whether or not you observed differences in reactivity between R = Cl and R = OMe for each set of reaction conditions. Draw the general reaction scheme with the R substituent for the aldehyde component, and include a separate table for each set of reaction conditions that you tried. Each table should have entries for each R group and the % yield for each substrate. Next, discuss how each set of reaction conditions differed, and comment on whether or not you observed any advantages with one set of conditions over the other. Conclusion: start with a brief statement of the overall success of the project. Then, recount the chemistry results, note whether or not you observed a substituent effect in the reaction, and explain which procedure is preferred. If one (or both) were not successful, provide a hypothesis as to what happened and why. Experimental (for each compound prepared; each group member should write one): first, draw the reaction performed, and below this put in a reaction table just like the ones you put in your notebook to organize reactants, mmol amounts, equivalents, etc. Below this, type up the experimental procedure for each Cannizzaro reaction in the same way that you record it in your notebook. Please also include the printouts for the IR and 1H NMR spectra that you generate for each product that you make (and please make sure these are properly labelled!). Be sure to assign all major peaks in the IR and all peaks in the NMR. For TLC, you may hand‐draw the TLC plate at the end of each procedure, write in the mobile phase used, and show Rf’s. Be sure to include in the procedure that you ran a TLC, what mobile phase you used, and comment on what you observed. Final Note on Overall Grade Please note that the overall grade for each individual on the team is solely based on the team’s overall grade for this project. As such, it is VITAL that each and every team member participates and shares in ALL ASPECTS of the work for this project. All team members should perform the searches, do the lab work, help compile and edit the in‐class PowerPoint presentation, as well as contribute to and edit the final lab write‐up. If there is a problem with a particular group member (and this is not anticipated), please bring this up with your instructor immediately.