Slides - Fondation Nanosciences
advertisement
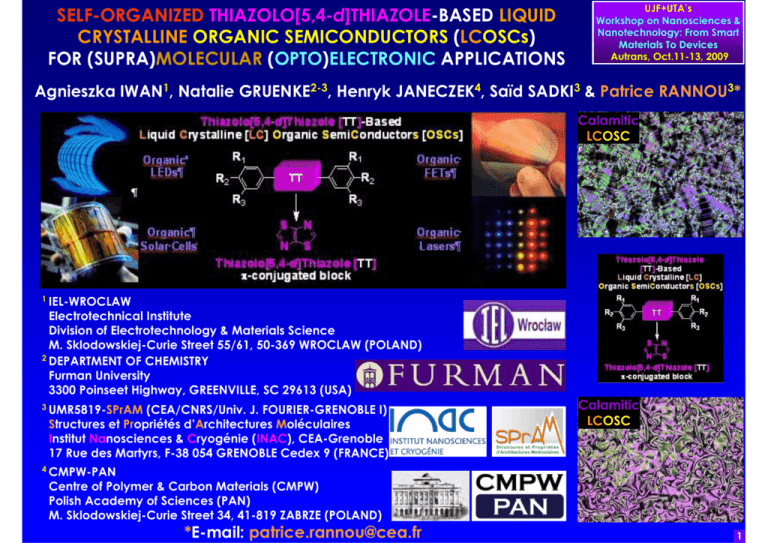
SELF-ORGANIZED THIAZOLO[5,4-d]THIAZOLE-BASED LIQUID CRYSTALLINE ORGANIC SEMICONDUCTORS (LCOSCs) FOR (SUPRA)MOLECULAR (OPTO)ELECTRONIC APPLICATIONS UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 Agnieszka IWAN1, Natalie GRUENKE2-3, Henryk JANECZEK4, Saïd SADKI3 & Patrice RANNOU3* Calamitic LCOSC 1 IEL-WROCLAW Electrotechnical Institute Division of Electrotechnology & Materials Science M. Sklodowskiej-Curie Street 55/61, 50-369 WROCLAW (POLAND) 2 DEPARTMENT OF CHEMISTRY Furman University 3300 Poinseet Highway, GREENVILLE, SC 29613 (USA) 3 UMR5819-SPrAM (CEA/CNRS/Univ. J. FOURIER-GRENOBLE I) Structures et Propriétés d’Architectures Moléculaires Institut Nanosciences & Cryogénie (INAC), CEA-Grenoble 17 Rue des Martyrs, F-38 054 GRENOBLE Cedex 9 (FRANCE) 4 CMPW-PAN Centre of Polymer & Carbon Materials (CMPW) Polish Academy of Sciences (PAN) M. Sklodowskiej-Curie Street 34, 41-819 ZABRZE (POLAND) *E-mail: patrice.rannou@cea.fr Calamitic LCOSC 1 OUTLINE •MOLECULAR vs. SUPRAMOLECULAR vs. ORGANIC (OPTO)ELECTRONICS UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 •STATE OF THE ART PERFORMANCES OF ORGANIC (OPTO)ELECTRONIC DEVICES •THIAZOLO[5,4-d]THIAZOLE-BASED OSCs &CALAMITIC/HEXACATENAR LCOSCs: SYNTHETIC ROUTE & LIBRARY Nem* •THIAZOLO[5,4-d]THIAZOLE-BASED OSCs & CALAMITIC/HEXACATENAR LCOSCs: SEC CHARACTERIZATIONS •THIAZOLO[5,4-d]THIAZOLE-BASED OSCs & CALAMITIC/HEXACATENAR LCOSCs: DSC & POM & XRD CHARACTERIZATIONS •THIAZOLO[5,4-d]THIAZOLE-BASED OSCs & CALAMITIC/HEXACATENAR LCOSCs: UV-Vis & PL CHARACTERIZATIONS •THIAZOLO[5,4-d]THIAZOLE-BASED OSCs & CALAMITIC/HEXACATENAR LCOSCs: CV & DPV CHARACTERIZATIONS •CONCLUSIONS, ONGOING/FUTURE STUDIES & ACKNOWLEDMENTS 2 MOLECULAR vs. SUPRAMOLECULAR vs. ORGANIC (OPTO)ELECTRONICS UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 MOLECULAR (OPTO)ELECTRONICS SUPRAMOLECULAR (OPTO)ELECTRONICS ORGANIC (OPTO)ELECTRONICS ~1-5nm ~5->100nm ~100nm->20µ µm SINGLE MOLECULES π-CONJUGATED MOLECULAR WIRES (OLIGOMERS) BOTTUM UP APPROACH Nature 408, 541 (2000). Science 300, 1384 (2003). Nat. Nanotech. 1, 173 (2006). Acc. Chem. Res. 41, 1731 (2008). Angew. Chem. Int. Ed. 48, 3911 (2009). THIN FILMS/SINGLE CRYSTAL SELF-ORGANIZED & OF OSCs HIERARCHIZED OSCs (MOLECULES, (MOLECULES, DENDRIMERS, DENDRIMERS, OLIGOMERS MACROMOLECULES, LCs & (CO)-POLYMERS) & SUPRAMOLECULES) ADVANCED CMOS PLATE-FORME Nature 419, 353 (2002). Nat. Mater. 3, 507 (2004). Chem. Commun. 3245 (2005). TOP DOWN APPROACH Nature 428, 911 (2004). 3 UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 STATE OF THE ART PERFORMANCES OF ORGANIC (OPTO)ELECTRONIC DEVICES: OLEDs/PLEDs, SOLAR CELLS, (SC)OFETs/PFETs & LASERS •OLEDs/PLEDs •(SC)OFETs/PFETs 40" OLED TV: Prototype 2006 MOLECULAR & MACROMOLECULAR π-CONJUGATED 11" OLED TV (XEL-1): Dec. 2007 ARCHITECTURES: Nature 397, 121 (1999). J.Mater.Chem. 10, 1471 (2000). Phys.StatusSolidi (a) 201, 1302 (2004). Adv. Chem.Soc.Rev. 37, 2543 (2008). Chem.Rev. 109, 897 (2009). Mater. 17, 2411 (2005). Rev.Mod.Phys. 78, 973 FUNCTIONAL Nature 459, 234 (2009). (2006). Mater.Today 10, 20 (2007). Adv.Mater. R-G-B-WHITE OLEDs/PLEDs 19, 1791 (2007). Chem.Rev. 107, 926 (2007). OSCs 5 -2 Chem.Rev. 107, 1066 (2007). Chem.Rev. 107, WITH LIFETIME UP TO 10 HOURS @1000 cd.m WITH TUNABLE 1296 (2007). Chem.Eur.J. 14, 4766 (2008). -1 WITH EFFICIENCY UP TO 110 lm.W Angew.Chem.Int.Ed. 47, 4070 (2008). Adv. (OPTO)ELECTRONICS Mater. 21, 1473 (2009). •LIGHT-EMITTING FEATURES p-TYPE SCOFETs: µh UP TO ~10-43.0 cm2.V-1.s-1 (SC)OFETs/PFETs n/p-TYPE OFETs: µh/e- UP TO ~5.0-6.0 cm2.V-1.s-1 Nat.Mater. 5, 605 (2006). Chem.Rev. 107, 1296 (2007). Adv. + n-TYPE PFETs: µe- UP TO ~0.9 cm2.V-1.s-1 Mater. 19, 1791 (2007). Adv.Funct.Mater. 17, 3421 (2007).Adv. Funct.Mater.19, 1728 (2009). Appl.Phys.Lett. 95, 103307 2009 "WET" / "DRY" p-TYPE PFETs: µh UP TO ~3.0 cm2.V-1.s-1 •SOLAR CELLS PROCESSING ROUTES •ORGANIC/PLASTIC LASERS R EMERGING FIELD OF ORGANIC/PLASTIC (OPTO)ELECTRONICS Adv.Funct.Mater. 11, 15 (2001). Mater.Today 7, 36 (2004). Mater.Today 10, 28 (2007). Mater.Today 10, 34 (2007). Adv.Funct.Mater. 18, 169 (2008). Adv.Mater. 21, 1323 (2009). Adv.Mater. 21, 1434 (2009). EPCE (UNDER AM1.5) UP TO 6.5% S.R. Forrest, "The path to ubiquitous and low-cost organic electronic appliances on plastic", Science 317, 222 (2007). Nat.Photon. 3, 297 (2009) Nature 428, 911 (2004). R R R n LPPP Rep.Prog.Phys. 63, 729 (2000). Mater.Today 7, 28 (2004). Chem.Rev. 107, 1272 (2007). J.Mater.Chem. 19, 7520 (2009) NO REPORT TO DATE OF AN ELECTRICALLY-DRIVEN ORGANIC/PLASTIC LASER 4 THIAZOLO[5,4-d]THIAZOLE-BASED OSCs & CALAMATIC/HEXACATENAR LCOSCs: SYNTHETIC ROUTE & LIBRARY J. Ephraim, Ber. 94, 1027 (1891). J.R. Jonhson & R. Ketcham, J. Am. Chem. Soc. 82, 2719 (1960). R1 R2 O C R3 yield ~15-50% S H H2N C C NH2 S dithioxamide R1 R2 R3 S N N S R1 UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 R2 R3 5 THIAZOLO[5,4-d]THIAZOLE-BASED OSCs & CALAMATIC/HEXACATENAR LCOSCs: SIZE EXCLUSION CHROMATOGRAPHY (SEC) CHARACTERIZATIONS UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 SEC Line: HP Chemstation 1100 SEC Column: 300*7.5mm PL-Gel Mixed-D 5µm/104 Å @ 313K Calibration Curve: 10 PS Narrow Standards (PL: Kit S-M2-10*) Eluent: THF, Flow Rate: 1ml.min-1, RI & UV-Vis Detection 20 µl Injection 6 THIAZOLO[5,4-d]THIAZOLE-BASED OSCs &CALAMATIC/HEXACATENAR LCOSCs: DIFFERENTIAL SCANNING CALORIMETRY (DSC), POLARIZED OPTICAL MICROSCOPY (POM) & XRD CHARACTERIZATIONS LCTT23-SmC @ 152.5°C 50µ µm UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 LCTT23-SmG @ 140.0°C 50µ µm LCTT23-SmF @ 148.0°C 50µ µm LC/LC* LASERS LC/LC* LASERS: TOWARDS APPLICATIONS IN LC/LC* LASER & COLOR INFORMATION TECHNOLOGY (CIT) UPON FURTHER REFINEMENT OF THE CHEMICAL STRUCTURE (R1/R2/R3) OF TT-BASED LCOSCs LCTT21 ISO->Nem @ 201.0°C Nem->SmC @ 200.0°C SmC->SmI @ 121.5°C SmI ->Cr @ 119.0°C LCTT21-SmC @ 125°C 90µ µm LCTT21-SmI @ 120°C 90µ µm LCTT31 ISO-> Nem* @ 217.5°C Nem*->G @ 145.0°C Nem* LCTT23 ISO->SmC @ 185.0°C SmC->SmF @ 150.0°C SmF->SmG @ 145.0°C SmG-> Cr @ 51.5°C R. Won, Nat.Photon. 2, 593 (2008). D. Graham-Rowe, Nat.Photon. 3, 183 (2009). LC/LC* CIT N. Tamaoki, Adv.Mater. 13, 1135 (2001). LCTT31-Nem* @ 210°C 25µ µm LCTT31-Nem* @ 200°C 90µ µm LCTT31-Nem* @ 160°C 90µ µm LCTT31-Nem* @ 180°C 90µ µm LCTT22 ISO-> SmC @ 222.5°C SmC ->Cr @ 192.5°C LCTT24 ISO ->SmA @ 265.0°C SmA->SmB @ 225.0°C SmB ->Cr @ 192.5°C LCTTs: J. Bartulin et al., Mol.Cryst.Liq.Cryst. 180, 297 (1990). Y.Z. Youssif & A.J.A. Hamdani, Liq.Cryst. 15, 451 (1993). 7 THIAZOLO[5,4-d]THIAZOLE-BASED OSCs & CALAMATIC/HEXACATENAR LCOSCs: UV-VIS ABSORPTION (UV-Vis) & PHOTOLUMINESCENCE SPECTROSCOPIC (PL) CHARACTERIZATIONS OPTOELECTRONIC FEATURES OF TT1: M.R. Pinto et al. , J.Photochem. Photobiol.A 143, 119 (2001). UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 THIAZOLO[5,4-d]THIAZOLE-BASED OSCs & CALAMATIC/HEXACATENAR LCOSCs: TOWARDS APPLICATIONS • "HIGH" OPTICAL BANG GAP IN VIOLET-BLUE OSCs: ~2.7eV< EgOpt. < ~3.2 eV LC OLEDs & • TUNABLE VIOLET-BLUE EMITTERS BY DESIGN LC/LC*LASERS • PL EMISSION TUNING OVER ~22 nm LC BY DESIGN & BY APPROPRIATE CHOICE OF OLEDs R1/R2/R3 FUNCTIONAL (ACCEPTING OR DONATING) GROUPS LC OLEDs: M. O’Neil & S.M. Kelly, Adv.Mater. 39, 4223 (2007). LC/LC* LASERS: R. Won, Nat.Photon. 2, 593 (2008). D. Graham-Rowe, Nat.Photon. 3, 183 (2009). LC/LC* LASERS 8 THIAZOLO[5,4-d]THIAZOLE-BASED OSCs & CALAMATIC/HEXACATENAR LCOSCs: CYCLIC VOLTAMMETRY (CV) & DIFFERENTIAL PULSE VOLTAMMETRY (DPV) CHARACTERIZATIONS UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 ELUMO/HOMO [eV]~ -5.1 - Epeak TOWARDS APPLICATIONS IN LC OLEDs, LC/LCOFETS & LC SOLAR CELLS UPON •FURTHER REFINEMENT OF THE CHEMICAL STRUCTURE (R1/R2/R3) OF TT-BASED LCOSCs •APPROPRIATE CHOICE OF ELECTRODES TT1 & OF DONNOR/ACCEPTOR COUPLES EgOpt.~ 3.16eV LC EgElectroch.~ 3.08 eV OLEDs CV/DPV: Indirect Estimation of HOMO/LUMO Levels S. Trasati, Pure&Appl.Chem. 58, 955 (1986). I. Polenc et al., J.Polym.Sci.Polym.Chem. LC 41, 1034 (2003). OFETs UPS/IPES vs. CV/DPV vs. DFT: (In)Direct Estimation of HOMO/LUMO Levels LC P.I. Djurovitch et al., Org.Electron. 10, 515 (2009). SOLAR CV/DPV Conditions: W.E./C.E.: Pt & Ref. E.: Ag/AgCl vs. Fc+/Fc -1 -2 -4 CELLS CV/DPV Scan rates: 100/10mV.s . ~10 -10 M in ACN (0.1 M TBAP). LC OLEDs: M. O’Neil & S.M. Kelly, Adv.Mater. 39, 4223 (2007). LC OFETs: Y. Shimizu et al., J.Mater.Chem. 39, 4223 (2007). LC Solar Cells: L. Schmidt-Mende et al. Science 293, 1119 (2001). 9 CONCLUSIONS, ONGOING/FUTURE STUDIES & ACKNOWLEDGMENTS UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 CONCLUSIONS •We have designed, synthesized and characterized a concise library of Thiazolo[5,4-d]Thiazole (TT)-based molecular Organic SemiConductors (OSCs) and Liquid Crystals (LCs) consisting of calamitic & hexacatenar π-conjugated thermotropic functional LCOSCs with an (opto)electronically active (lath-shaped) TT core. •The appropriate choices of R1/R2/R3 functional groups of different stiffness, chemical or electronic natures allow for a rich mesomorphism [Nem, SmA/B/C/F/G/I and Nem* mesophases] and for tunable on-demand optoelectronic properties [λ λmax [nm], λem. [nm], Band Gap Eg [eV], HOMO and LUMO levels [eV]] of great scope for testing their potentials as [stimuli responsive: temperature, pH, light …] active layers of new generations of supramolecular (opto)electronic devices [LEDs, Solar Cells, Lasers & FETs]. Natalie Gruenke Agnieszka Iwan Saïd Sadki Henryk Janeczek ACKNOWLEDGEMENTS: FUNDING/SUPPORTS •Univ. J. FOURIER + FURHMAN Univ. [2009] •CNRS/PAN [2009-2010] "Project N°22529" ONGOING & FUTURE STUDIES • Developing Libraries of p-type & n-type Calamitic/Tetracatenar/Hexacatenar Thiazolo[5,4-d]Thiazole-based LCOSCs. •Studying the impact of geometrical/electrical confinements [within operating devices] on - the mesomorphism & structural organization - the (opto)electronic features of Thiazolo[5,4-d]Thiazole-based Calamitic/Tetracatenar/Hexacatenar LCOSCs. 10 CONVENTIONAL OSCs vs. FUNCTIONAL THERMOTROPIC π-CONJUGATED LC OSCs "CONVENTIONAL" OSCs •FIXED "STATIC " MULTI-SCALE ORDER/DISORDER • STRUCTURAL ORGANIZATIONS vs. ELECTRONIC TRANSPORT PROPERTIES RELATIONSHIPS Nobel Prize in Chemistry 2000! S.R. Forrest, Nature 428, 911 (2004). UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 FUNCTIONAL THERMOTROPIC π-CONJUGATED LCOSCs • TUNABLE (F(T)) & "DYNAMIC" (LC STATE) MULTI-SCALE ORDER/DISORDER + SELF-HEALING ABILTY STRUCTURAL ORGANIZATIONS vs. ELECTRONIC TRANSPORT PROPERTIES RELATIONSHIPS • T. Kato et al., Angew.Chem.Int.Ed.45, 38 (2006). T. Kato et al., Chem.Commun., 729 (2009). J.W. Goodby et al., Liq.Cryst. 36, 567 (2009). W. Pisula et al., Macromol.RapidCommun. 30, 1179 (2009). •OSCs: IMPORTANTES DATES •LCs & LC OSCs: IMPORTANTES DATES 1977: DISCOVERY OF THE 1888: DISCOVERY OF CHOLESTERIC LCs BY (AUSTRIAN CONDUCTING POLYMERS BOTANIST) F. REINITZER & (GERMAN PHYSICIST) O. LEHMANN 1983: 1st PFETs st REPORT ON CALAMITIC LCs BY D. VORLÄNDER 1907: 1 st 1986: 1 EFFICIENT OLEDs 1977: 1st REPORT ON DISCOTIC LCs BY S. CHANDRASEKHAR & SOLAR CELLS 1993: FAST PHOTOCONDUCTION IN π-CONJUGATED 1990: 1st PLEDs 1994 DISCOTIC LCOSCs BY D. HAARER et al. 1997: OLED DISPLAYS 1996: FAST PHOTOCONDUCTION IN π-CONJUGATED 2007: TV AM-OLEDs 1997 SMECTIC LCOSCs BY J.-I. HANNA et al. 11 UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 ELECTRONIC TRANSPORT PROPERTIES OF LCOSCs: PART I) DISCOVERY OF THE FAST PHOTOCONDUCTION IN π-CONJUGATED DISCOTIC & SMECTIC LCOSCs 1996-1997 O S S S S S 2-(dodecyloxy)-6-(4-octylphenyl)naphthalene Cr 79°C SmB 100°C SmA 121 Iso S 1993-1994 2,3,6,7,10,11-hexakis(hexylthio)triphenylene Cr 62°C H (Colh*) 70°C Dh (Colhex*) 93°C Iso O N S S 1997 6-(dodecylthio)-2-(4-(heptyloxy)phenyl)benzo[d]thiazole Cr 90°C SmA 100°C Iso PHOTOCONDUCTION IN LCOSCs R.J. Bushby & O.R. Lozman, Curr.Opin.SolidStateMater. Sci. 6, 569 (2002). M. O’Neill & S.M. Kelly, Adv.Mater. 15, 1135 (2003). M. Funahashi et al., Struct.Bond. 128, 151 (2008). M. Funahashi & J. Hanna, Jpn.J.Appl.Phys. 335, L703 (1996). By TOF: µh [SmA] up to 0.001 cm2.V-1.s-1 M. Funahashi & J. Hanna, Phys. Rev. Lett. 78, 2184 (1997). By TOF: µh [SmA] up to 0.005 cm2.V-1.s-1 D. Adam et al., Nature 371, 141 (1994). By TOF: µh= µe- [H] up to 0.1cm .V .s 2 -1 -1 D. Adam et al., Phys.Rev.Lett. 70, 457 (1993). By TOF: µh= µe- up to 0.001 cm2.V-1.s-1 M. Funahashi & J. Hanna, Mol.Cryst.Liq.Cryst. 304, 429 (1997). M. Funahashi & J. Hanna, Appl.Phys.Lett. 71, 602 (1997). By TOF: µh=µ µe- [SmB] up to 0.0016 cm2.V-1.s-1 By TOF: µh=µ µe- [SmA] up to 0.00025 cm2.V-1.s-1 12 ELECTRONIC TRANSPORT PROPERTIES OF LCOSCs: PART II) CHARGE CARRIER (HOLES/ELECTRONS) MOBILITY AS PROBED BY THE PR-TRMC vs. TOF vs. LCOFET UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 •3D: ELECTRONIC TRANSPORT WITHIN THE BULK •CONTACT/ELECTRODE-LESS TECHNIQUE •E- BEAM DOPING: NANOSECOND PULSE OF 3 MeV ELECTRONS Σµ= (µh + µe-) [cm2.V-1.s-1] PR-TRMC J.M. Warman et al., Chem.Mater. 16, 4600 (2004). •3D: ELECTRONIC TRANSPORT WITHIN THE BULK •CONTACT/WAVELENGTH-DEPENDENT •PHOTO-DOPING: PHOTO-GENERATED CHARGE CARRIERS µh & µe- [cm2.V-1.s-1] TOF H. Iino & J. Hanna, Opto-Electron.Rev. 13, 295 (2005). M. Funahashi et al., Struct.Bond. 128, 151 (2008). LC OFET Source LC OSCs Gate Dielectric Gate Drain •2D: ELECTRONIC TRANSPORT CONFINED @ THE GATE DIELECTRIC/OSC INTERFACE •CONTACT-DEPENDENT •FIELD-EFFECT DOPING: ELECTRICALLY GENERATED CHARGE CARRIERS µh & µe- [cm2.V-1.s-1] Y. Shimizu et al., J. Mater. Chem. 39, 4223 (2007). 13 ELECTRONIC TRANSPORT PROPERTIES OF LCOSCs IN OFET CONFIGURATIONS: STATE OF THE ART CHARGE CARRIER (HOLES & ELECTRON) MOBILITY VALUES IN DISCOTIC & SMECTIC LCOSCs UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 Y. Shimizu et al., "Mesophase semiconductors in field effect transistors" J.Mater.Chem. 39, 4223 (2007). DISCOTIC p-Type LCOSCs SMECTIC n-Type LCOSCs DISCOTIC n-Type LCOSCs SMECTIC p-Type LCOSCs p-Type LCPSCs 14 THERMOTROPIC LC OSCs IN LC OFET CONFIGURATIONS: NEW OPPORTUNITIES/PLAYGROUNDS FOR FUNDAMENTAL STUDIES ON LCOSCs Source Discotic LCOSC LCOFET Top-Cover Glass LC OSCs Gate Dielectric Gate Drain Source UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 LC OSCs Gate Dielectric Gate Drain LCOSCs’s CONFINEMENT (@ TWO DIFFERENT LENGTHSCALES) WITHIN THE CHANNEL OF A LC OFET -IN BETWEEN S & D (ca. µm-SCALE): GEOMETRICAL EFFECTS -IN THE BEWTEEN THE GATE DIELECTRIC/AIR OR THE GATE DIELECTRIC/TOP-COVER GLASS INTERFACE (DOWN TO THE nm-SCALE): GEOMETRICAL EFFECTS DISCOTIC LC OSCs SMECTIC LC OSCs TOROIDAL DEFECTS E. Grelet & H. Bock, Europhys. Lett. 73, 712 (2006) Calamitic LCOSC Calamitic LCOSC P.O. Mouthuy et al., NanoLett. 7, 2627 (2007) M.C. Choi et al., Proc.Nat.Acad.Sci. 101, 17340 (2004). D.K. Yoon et al., Nat.Mater. 6, 866 (2007). LCOFET Y. Shimizu et al., J.Mater.Chem. 39, 4223 (2007). J.C. Maunoury et al., Adv.Mater. 19, 805 (2007) 15 THERMOTROPIC LCOSCs IN LC OFET CONFIGURATIONS: 5 OPEN QUESTIONS (Q1-5) UJF+UTA’s Workshop on Nanosciences & Nanotechnology: From Smart Materials To Devices Autrans, Oct.11-13, 2009 LCOSCs’s CONFINEMENT (@ TWO DIFFERENT LENGTHSCALES) WITHIN THE (OPTO)ELECTRONICALLY ACTIVE CHANNEL OF AN LC OFET UNDER OPERATION: •MICRO & NANO-CONFINEMENTS INDUCED BY GEOMETRICAL CONSTRAINTS + •ELECTRICAL CONFINEMENTS INDUCED BY THE FIELD-EFFECT DOPING @ THE QUASI-2D LCOSC/GATE DIELECTRIC INTERFACE Q1: NEW/DIFFERENT MESOMORPHISM FOR (KNOWN) LCOSCs? Discotic LCOSC Calamitic LCOSC Calamitic LCOSC Y. Shimizu et al., LCOFET Q2: A CONTROL OVER THE HOMOGENEOUS vs. HOMEOTROPIC ALIGNMENT OF AN (OPTO)ELECTRONICALLY ACTIVE LCOSC LAYER? Q3: A WAY TO STABILIZE MONO-DOMAINS OF LCOSCs? Q4: IMPACT ON THE DYNAMIC SELF-ASSEMBLY, SELF-HEALING & (OPTO)ELECTRONIC FEATURES OF LCOSCs? Q5: IMPACT ON THE (CASCADE OF) INTRICATE (OPTO)ELECTRONIC PROCESSES @ WORK WITHIN LCOSCsBASED OFETs Q1-5 : GENERIC ISSUES FOR LCOSCs-BASED LEDs LCOFET MULTI-LAYER & BULK-HETEROJUNCTION SOLAR CELLS J.Mater.Chem. 39, 4223 (2007). LASERS 16








