TYPES OF INFORMATION FROM NMR SPECTRUM
advertisement
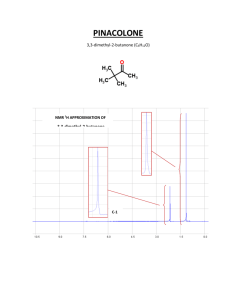
TYPES OF INFORMATION FROM NMR SPECTRUM 1. Each different type of hydrogen gives a peak or group of peaks (multiplet). 2. The chemical shift (δ, in ppm) gives a clue as to the type of hydrogen generating the peak (alkane, alkene, benzene, aldehyde, etc.) 3. The integral gives the relative numbers of each type of hydrogen. 4. Spin-spin splitting gives the number of hydrogens on adjacent carbons. 5. The coupling constant J also gives information about the arrangement of the atoms involved. CHEMICAL SHIFT DIAMAGNETIC ANISOTROPY SHIELDING BY VALENCE ELECTRONS Diamagnetic Anisotropy Applied magnetic field induces circulation of valence electrons - this generates a magnetic field that opposes the applied field. valence electrons shield the nucleus from the full effect of the applied field magnetic field lines Bo applied B induced (opposes Bo) PROTONS DIFFER IN THEIR SHIELDING All different types of protons in a molecule have a different amounts of shielding. They all respond differently to the applied magnetic field and appear at different places in the spectrum. DOWNFIELD deshielded protons appear here. SPECTRUM UPFIELD shielded protons appear here. PEAKS ARE MEASURED RELATIVE TO TMS Rather than measure exact position of a peak, we measure how far downfield it is shifted from TMS. reference compound tetramethylsilane “TMS” CH3 CH3 Si CH3 CH3 Highly shielded protons appear way upfield. TMS shift in Hz downfield n 0 THE CHEMICAL SHIFT The shifts from TMS in Hz are bigger in higher field instruments (300 MHz, 500 MHz) than they are in the lower field instruments (100 MHz, 60 MHz). We can adjust the shift to a field-independent value, the “chemical shift” in the following way: parts per million chemical = shift δ shift in Hz = = ppm spectrometer frequency in MHz This division gives a number independent of the instrument used. A particular proton in a given molecule will always have the same chemical shift (constant value). NMR Correlation Chart -OH -NH DOWNFIELD DESHIELDED UPFIELD SHIELDED CHCl3 , H TMS 12 11 10 9 8 7 6 H RCOOH RCHO C=C 5 4 CH2F CH2Cl CH2Br CH2I CH2O CH2NO2 3 2 CH2Ar CH2NR2 CH2S C C-H C=C-CH2 CH2-CO 1 0 δ (ppm) C-CH-C C C-CH2-C C-CH3 Ranges can be defined for different general types of protons. This chart is general, the next slide is more definite. DESHIELDING AND ANISOTROPY Three major factors account for the resonance positions (on the ppm scale) of most protons. 1. Deshielding by electronegative elements. 2. Anisotropic fields usually due to pi-bonded electrons in the molecule. 3. Deshielding due to hydrogen bonding. DESHIELDING BY ELECTRONEGATIVE ELEMENTS DESHIELDING BY AN ELECTRONEGATIVE ELEMENT δ- Cl δ+ C δ- electronegative element H δ+ Chlorine “deshields” the proton, it takes valence electron density away from carbon, which in turn takes more density from hydrogen deshielding the proton. NMR CHART “deshielded” protons appear downfield More shielded protons appear upfield deshielding moves proton resonance to lower field Electronegativity Dependence of Chemical Shift Dependence of the Chemical Shift of CH3X on the Element X Compound CH3X Element X Electronegativity of X Chemical shift δ most deshielded CH3F CH3OH CH3Cl CH3Br CH3I CH4 (CH3)4Si F O Cl Br I H Si 4.0 3.5 3.1 2.8 2.5 2.1 1.8 4.26 3.40 3.05 2.68 2.16 0.23 0 TMS deshielding increases with the electronegativity of atom X Substitution Effects on Chemical Shift most deshielded most deshielded CHCl3 CH2Cl2 CH3Cl 7.27 5.30 3.05 -CH2-Br 3.30 ppm -CH2-CH2Br 1.69 The effect increases with greater numbers of electronegative atoms. -CH2-CH2CH2Br 1.25 ppm The effect decreases with incresing distance. ANISOTROPIC FIELDS DUE TO THE PRESENCE OF PI BONDS The presence of a nearby pi bond or pi system greatly affects the chemical shift. Benzene rings have the greatest effect. Ring Current in in Benzene Circulating H Bo H π electrons Deshielded fields add together Secondary magnetic field generated by circulating π electrons deshields aromatic protons ANISOTROPIC FIELD IN AN ALKENE protons are deshielded Deshielded fields add H H shifted downfield C=C H Bo H secondary magnetic (anisotropic) field lines HYDROGEN BONDING HYDROGEN BONDING DESHIELDS PROTONS R O H H O R H O R The chemical shift depends on how much hydrogen bonding is taking place. Alcohols vary in chemical shift from 0.5 ppm (free OH) to about 5.0 ppm (lots of H bonding). Hydrogen bonding lengthens the O-H bond and reduces the valence electron density around the proton - it is deshielded and shifted downfield in the NMR spectrum. SOME MORE EXTREME EXAMPLES O H O C R R C O H O Carboxylic acids have strong hydrogen bonding - they form dimers. With carboxylic acids the O-H absorptions are found between 10 and 12 ppm very far downfield. H3C O O H O In methyl salicylate, which has strong internal hydrogen bonding, the NMR absortion for O-H is at about 14 ppm, way, way downfield. Notice that a 6-membered ring is formed. SPIN-SPIN SPLITTING SPIN-SPIN SPLITTING Often a group of hydrogens will appear as a multiplet rather than as a single peak. Multiplets are named as follows: Singlet Doublet Triplet Quartet Quintet Septet Octet Nonet This happens because of interaction with neighboring hydrogens and is called SPIN-SPIN SPLITTING. 1,1,2-Trichloroethane The two kinds of hydrogens do not appear as single peaks, rather there is a “triplet” and a “doublet”. integral = 2 Cl H H C C Cl Cl H integral = 1 triplet doublet The subpeaks are due to spin-spin splitting and are predicted by the n+1 rule. n+1 RULE 1,1,2-Trichloroethane integral = 2 Cl H H C C Cl integral = 1 Cl H Where do these multiplets come from ? Ö.. interaction with neighbors this hydrogen’s peak is split by its two neighbors these hydrogens are split by their single neighbor H H H H C C C C H two neighbors n+1 = 3 triplet H one neighbor n+1 = 2 doublet MULTIPLETS singlet doublet triplet quartet quintet sextet septet EXCEPTIONS TO THE N+1 RULE IMPORTANT ! 1) Protons that are equivalent by symmetry usually do not split one another X CH2 CH2 Y X CH CH Y no splitting if x=y 2) no splitting if x=y Protons in the same group usually do not split one another H C H H H or C H more detail later SOME COMMON PATTERNS SOME COMMON SPLITTING PATTERNS X CH CH Y CH3 CH (x=y) CH2 CH X CH2 CH2 Y (x=y) CH3 CH2 CH3 CH CH3 SOME EXAMPLE SPECTRA WITH SPLITTING NMR Spectrum of Bromoethane Br CH2CH3 NMR Spectrum of 2-Nitropropane H CH3 C CH3 + N O O- 1:6:15:20:16:6:1 in higher multiplets the outer peaks are often nearly lost in the baseline NMR Spectrum of Acetaldehyde O CH3 C offset = 2.0 ppm H INTENSITIES OF MULTIPLET PEAKS PASCAL’S TRIANGLE PASCALíS TRIANGLE Intensities of multiplet peaks 1 The interior 1 1 entries are the sums of 1 2 1 the two numbers 1 3 3 1 immediately above. 1 4 6 4 1 1 5 10 10 5 1 1 6 15 20 15 6 1 1 7 21 35 35 21 7 1 singlet doublet triplet quartet quintet sextet septet octet THE COUPLING CONSTANT THE COUPLING CONSTANT H H J C C H J J H H J J J The coupling constant is the distance J (measured in Hz) between the peaks in a multiplet. J is a measure of the amount of interaction between the two sets of hydrogens creating the multiplet. NOTATION FOR COUPLING CONSTANTS The most commonly encountered type of coupling is between hydrogens on adjacent carbon atoms. 3J H H C C This is sometimes called vicinal coupling. It is designated 3J since three bonds intervene between the two hydrogens. Another type of coupling that can also occur in special cases is 2J or geminal coupling H ( most often 2J = 0 ) C H Geminal coupling does not occur when 2J the two hydrogens are equivalent due to rotations around the other two bonds. SOME REPRESENTATIVE COUPLING CONSTANTS H H vicinal C C 6 to 8 Hz three bond 3J 11 to 18 Hz three bond 3J 6 to 15 Hz three bond 3J 0 to 5 Hz two bond H C C trans H H H cis C C H C geminal H Ha x Hax,Hax = 8 to 14 He q He q 2J Ha x Hax,Heq = 0 to 7 Heq,Heq = 0 to 5 three bond 3J OVERVIEW TYPES OF INFORMATION FROM THE NMR SPECTRUM 1. Each different type of hydrogen gives a peak or group of peaks (multiplet). 2. The chemical shift (δ, in ppm) gives a clue as to the type of hydrogen generating the peak (alkane, alkene, benzene, aldehyde, etc.) 3. The integral gives the relative numbers of each type of hydrogen. 4. Spin-spin splitting gives the number of hydrogens on adjacent carbons. 5. The coupling constant J also gives information about the arrangement of the atoms involved.