BEETROOT COURSEWORK PRACTICALS
advertisement

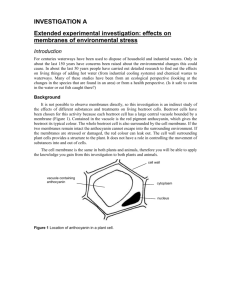
BEETROOT COURSEWORK - temperature or ethanol concentration PLANNING: The independent variable is the factor that you control. Thus you need to include full details of how you set about ensuring that the values you state are as accurate and reliable as possible. So: Temperature – use water-baths and measure the actual temperature allow enough time for the ‘ingredients’ to reach the indicated temperature – state in minutes how long. NB ‘Room Temperature’ does not exist in AS coursework – but you can use a water-bath with tap-water, by all means….. Volume - what volumes of liquids are you going to use? Why? How are you going to measure them? Why? Beetroot - How much are you going to use? Why? How are you going to cut them? Why? Is the beetroot uniform? How are you going to randomise your beetroot disks? What about genetic / growing / storage variations in the beetroot? Reaction vessels - What are you going to react the beetroot in? Why? Time - How long are you going to react the beetroot for? Why did you choose this time? How are you going to measure it? How are you going to ensure each replicate is the same? Range - what range of readings are you going to take? How did you decide on this? Will it be sufficient for a reliable result? Concentration - Range sufficient? Why chose this? Control - This will, of course, be part of the range of results taken, and so no separate control will be needed. The dependent variable is the one you measure. So: Absorbance - What are you going to measure this with? Why did you decide to use a Harris Digital Colorimeter, set up using filter 5 (yellow-green, peak absorbance at 550 nm)? How to you propose to calibrate it? What is the volume you use in each cuvette? How will you measure this? Replicates - How many readings are you going to generate per set of results? Clearly, you realise that the minimum is going to be 5. How many replicates are you intending to use? Can you combine your results with any other groups? Are you going to average them? Why? DIAGRAM: Have you done one? In pencil? Labelled? COLLECTION OF DATA Do ensure that you have collected your data on a decent bit of paper! These results need to be included in your final submission (as an appendix), as well as the neat, fully titled, wordprocessed table(s) in the ‘Results’ section of your report. Ensure your results are taken to an appropriate degree of accuracy and that you have enough in each set (min 5) and enough repetitions (min 3). You can pool results with others and average them, without fear, providing you state that they are pooled data. GRAPHING DATA: Titled. Lines labelled. Axes right way round – independent variable on the X axis, and fully labelled -with units! Most of graph paper area used – don’t plot on computer – the exam board don’t like them! Line of best fit optional; ‘joining up the dots’ = compulsory! Take care not to extrapolate back to the origin, unless you are certain that that is correct (it usually is not!) Error bars might be a worthwhile inclusion – if a histogram/bar chart is used. Calculations. As well as a bit of averaging, you might well be able to calculate a rate or two – anything (simple!) like this helps to give an air of authority to your conclusions. INTERPRETING Describe the pattern of your results – using actual numbers! This seems pointless and even offensive, given that the examiner can read and interpret the graph as well as you can. But do it! Conclusions need to be detailed. This means that you have to give an interpretation of your results. In this case, the purple pigment (probably betanin) is found in the vacuole in the centre of the cell. It is water-soluble. It is surrounded by both the vacuole and the cell membranes. The cellulose cell wall on the outside of the cell is fully permeable and so no barrier to the egress (= release) of the pigment. The pigment comes out because the two membranes are damaged. How? Membranes contain c.30% phospholipid (soluble in ethanol) and c.70% protein (denatured by high temps). So your account should explain what happens. The pigment is heat labile i.e. is denatured by heat – turning to a yellow chemical. Since the colorimeter is set to measure absorbance at one wavelength, a change in colour will result in a lower absorbance at that wavelength and so the numbers decrease. How does the pigment leave the cell (and why?)? The concentration outside the cell is low – so diffusion will be important. What factors affect the rate of diffusion? Did you leave the beetroot in the experiment for the optimum time? What would happen with (much) longer and shorter times? [See handout on Beetroot pigments] ANALYSING The main source of error in any biological experiment is usually the natural variation of living things. What did you do to ensure that this variation was minimised? The apparatus is more than accurate enough – but what about the disks themselves? ‘Experimental error’ counts for nothing – unless you detail the cause of the error and (better) indicate how the experiment could have been improved to reduce/eliminate the error that you have identified. Clearly, even with pooled class results only a limited range of results were obtained; what should be done to ensure that the results were reliable, repeatable and applicable to other situations? Simply repeating the experiment with the same apparatus and the same range of results will improve the reliability but not the accuracy. For that, the experiment must be modified – perhaps by using different apparatus (which we might not have – such as a more accurate colorimeter, which measures light over a much narrower range of wavelengths) Your anomalous results (or the class average’s) must be indicated on the graph(s) then seek to explain them – i.e. what is the most probable cause(s) of these results? Once again ‘Because that idiot XXXX did it’ won’t get any credit at all! IHW October 2003