Solubility Curve lab.doc
advertisement
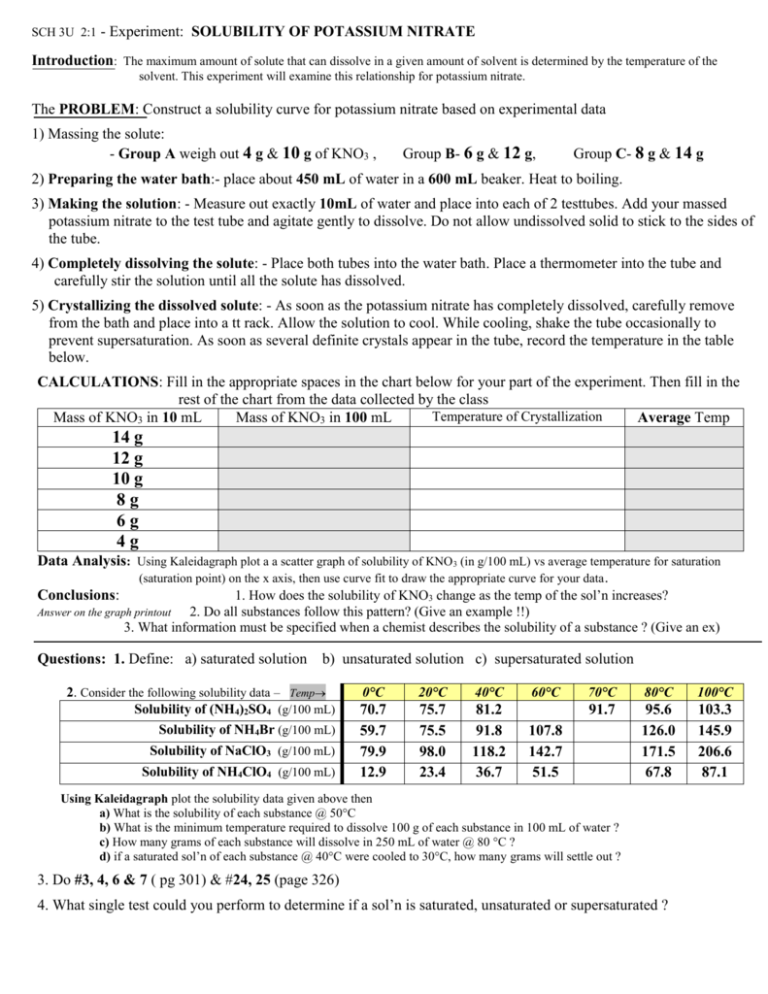
SCH 3U 2:1 - Experiment: SOLUBILITY OF POTASSIUM NITRATE Introduction: The maximum amount of solute that can dissolve in a given amount of solvent is determined by the temperature of the solvent. This experiment will examine this relationship for potassium nitrate. The PROBLEM: Construct a solubility curve for potassium nitrate based on experimental data 1) Massing the solute: - Group A weigh out 4 g & 10 g of KNO3 , Group B- 6 g & 12 g, Group C- 8 g & 14 g 2) Preparing the water bath:- place about 450 mL of water in a 600 mL beaker. Heat to boiling. 3) Making the solution: - Measure out exactly 10mL of water and place into each of 2 testtubes. Add your massed potassium nitrate to the test tube and agitate gently to dissolve. Do not allow undissolved solid to stick to the sides of the tube. 4) Completely dissolving the solute: - Place both tubes into the water bath. Place a thermometer into the tube and carefully stir the solution until all the solute has dissolved. 5) Crystallizing the dissolved solute: - As soon as the potassium nitrate has completely dissolved, carefully remove from the bath and place into a tt rack. Allow the solution to cool. While cooling, shake the tube occasionally to prevent supersaturation. As soon as several definite crystals appear in the tube, record the temperature in the table below. CALCULATIONS: Fill in the appropriate spaces in the chart below for your part of the experiment. Then fill in the rest of the chart from the data collected by the class Temperature of Crystallization Mass of KNO3 in 10 mL Mass of KNO3 in 100 mL Average Temp 14 g 12 g 10 g 8g 6g 4g Data Analysis: Using Kaleidagraph plot a a scatter graph of solubility of KNO3 (in g/100 mL) vs average temperature for saturation (saturation point) on the x axis, then use curve fit to draw the appropriate curve for your data . Conclusions: 1. How does the solubility of KNO3 change as the temp of the sol’n increases? 2. Do all substances follow this pattern? (Give an example !!) 3. What information must be specified when a chemist describes the solubility of a substance ? (Give an ex) Answer on the graph printout Questions: 1. Define: a) saturated solution b) unsaturated solution c) supersaturated solution 2. Consider the following solubility data – Temp Solubility of (NH4)2SO4 (g/100 mL) Solubility of NH4Br (g/100 mL) Solubility of NaClO3 (g/100 mL) Solubility of NH4ClO4 (g/100 mL) 0°C 20°C 40°C 70.7 59.7 79.9 12.9 75.7 75.5 98.0 23.4 81.2 91.8 118.2 36.7 60°C 70°C 80°C 100°C 91.7 95.6 126.0 171.5 67.8 103.3 145.9 206.6 87.1 107.8 142.7 51.5 Using Kaleidagraph plot the solubility data given above then a) What is the solubility of each substance @ 50°C b) What is the minimum temperature required to dissolve 100 g of each substance in 100 mL of water ? c) How many grams of each substance will dissolve in 250 mL of water @ 80 °C ? d) if a saturated sol’n of each substance @ 40°C were cooled to 30°C, how many grams will settle out ? 3. Do #3, 4, 6 & 7 ( pg 301) & #24, 25 (page 326) 4. What single test could you perform to determine if a sol’n is saturated, unsaturated or supersaturated ?