The Rate of chemical reactions
advertisement
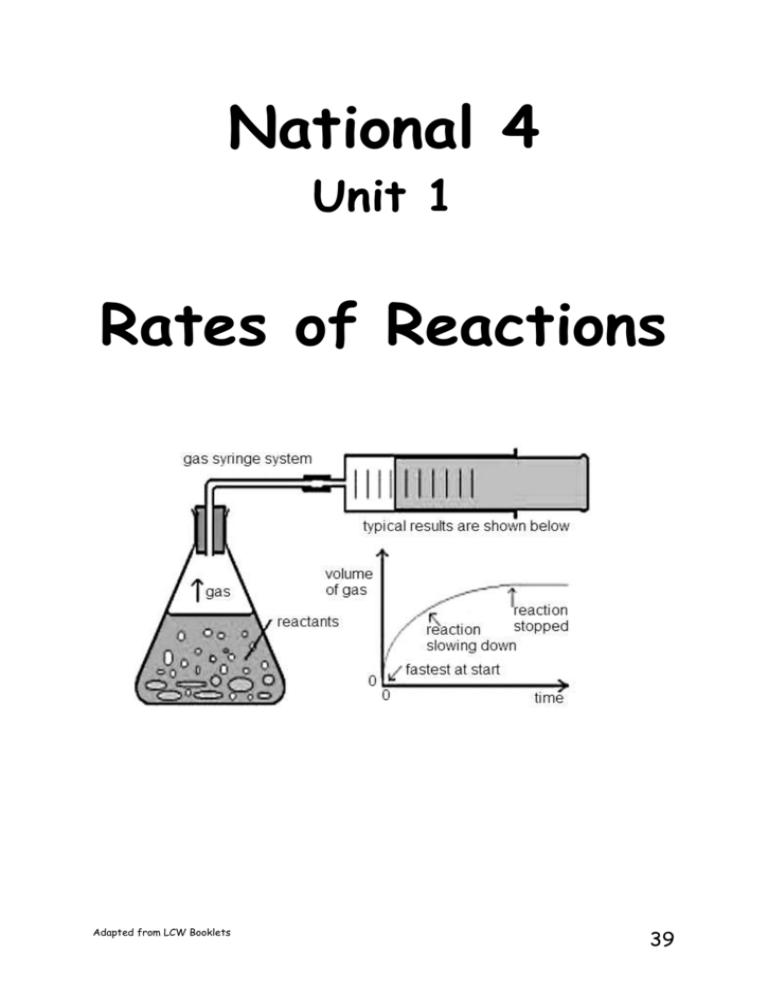
National 4 Unit 1 Rates of Reactions Adapted from LCW Booklets 39 The Rate of chemical reactions Learning intention Describe the rate of a chemical reaction as the speed that the reactants are used up or the speed that the products are made. Read Some reactions go faster than others. An explosion is an extremely fast reaction, making toast is a reaction that happens in minutes while a car rusting or a body rotting will take years. The rate of a reaction is a measure of how fast or slow a reaction proceeds. Your teacher might demonstrate some reactions for you (demo 1.24). Write a short description of each reaction making sure you include information about the rate of each reaction. Adapted from LCW Booklets 40 Success task Copy the table below and complete it by ticking the correct boxes. Look at all the reactions before you start ticking the boxes. Chemical reaction Reaction speed slow medium fast hydrogen exploding a car rusting a cake baking coal burning crude oil forming firing caps in a toy gun Adapted from LCW Booklets 41 Measuring the Rate of a Reaction Learning intention Describe how to measure the rate of a chemical reaction The rate of a reaction can be measured by monitoring how quickly a product is made or how quickly a reactant is used up. In S2 you completed some experiments where you monitored the rates of some reactions and discovered some things you could change to speed up these reactions. Discuss with your partner what you remember about what you learned in S2. Draw a spider diagram of what you both can recall. Reaction: When Calcium Carbonate (marble chips) reacts with hydrochloric acid the products formed are calcium carbonate, water and carbon dioxide gas. Think! How would you measure the rate of this reaction? How would you know if it was a fast or a slow reaction? Discuss this with your partner and come up with an answer. Adapted from LCW Booklets 42 Your teacher will now demonstrate how you can measure the rate of this reaction. Write a heading and try the work below. 1. What the reactants in the above reaction? 2. What are the products in the above reaction? 3. How was the rate of this reaction followed during the reaction? 4. Write a word equation for the above reaction. Extension Try to think of another way that we could measure the rate of this reaction (think of the marble chips !). Describe how you would do this. Adapted from LCW Booklets 43 Success task Copy and complete the following sentence. The rate of a reaction can be measured by the time take for a _________ to be used up or a __________ to form. Adapted from LCW Booklets 44 Collision Theory of Reacting Particles Learning intention Describe how particle size affects the rate of a reaction For any reaction to occur the reactants must collide into each other. They must be in contact to allow their atoms to react and form new substances. This is called Collision Theory. Anything that is done in a reaction to help particles/atoms to collide together will speed it up. Your teacher will show you more about collision theory using multimedia science school. Write down what happens to the atoms when we change each of the factors. We will now investigate these 3 factors which can affect the speed of a reaction. Compare your experimental findings with the ones you have made using multimedia science school. Adapted from LCW Booklets 45 Particle size and the speed of reactions Read The speed of a reaction can be affected by using different sizes of particles. Your teacher will demonstrate some reactions for you Burning Burning Burning Burning magnesium ribbon and magnesium powder. a lump of coal and powdered coal iron wire, steel wool and iron powder normal sugar and icing sugar. After watching the demonstration, copy and complete the following paragraph using words from the word list. Remember to read the paragraph before you start to complete it. WORD LIST – flame faster branch powder Slowly faster A lump of coal or wood will burn _________ in air. If we break up the lump, the reaction is _________. Eg, wood shavings burn ________ than a __________ from a tree. If we use a __________. The reaction is even faster, particularly when it is blown through a ________. Adapted from LCW Booklets 46 Write a heading and try the work below. Collect experiment card 1.26, and do the experiment. Answer the following questions 1. What did you see that told you a chemical reaction happened when you added the tablet to the acid? 2. How did you know which reaction was the fastest? 3. Make a list of two things you did to make sure the two experiments were fair. 4. Copy and complete the sentence below. The __________ the particles the __________ the reaction. Adapted from LCW Booklets 47 Extension 1.Which will burn faster - small pieces of coal or large lumps of coal? 1. Which will burn faster - a wooden log or sawdust? 2. Which will cook faster - small potatoes or large potatoes? 3. Dr Webster was running late and wanted her barbecue to light quickly. She had a choice between using charcoal lumps or broken pieces of charcoal she found at the bottom of an old bag of charcoal. Which would be the best to use and why? 4. Explain why it can be extremely dangerous to light a match in an icing sugar factory but not so dangerous in a sugar lump factory. Adapted from LCW Booklets 48 Another Way of Following the Rate of Reactions Using Graphs Learning intention Describe how a graph can inform us about the rate of a reaction. Read So far we have been observing the rates of chemical reactions by looking at how quickly products are formed. This is what we did in S2. However, we can also collect data from these reactions and line graphs can be drawn from the data. These line graphs can also tell us information about the rates of reactions. Adapted from LCW Booklets 49 Effect of Particle Size: Following the Rate of Reaction Your teacher will now take you through a reaction where we can follow the rate of the reaction by looking at a graph of the results. The experiment will be carried out with Marble chips Crushed marble chips Time / Minutes 0 1 2 3 4 5 6 7 8 9 10 11 12 Adapted from LCW Booklets Volume CO2 Chips Volume of CO2 Powder 50 Success task The results can then be used to draw a line graph. You can draw both lines on the same graph Draw each line a different colour. Complete the Line Graph Success Criteria sheet Answer the following questions 1. Which reaction was faster ? 2. what evidence from our graph tells us this ? 3. if the experiment was repeated with a lump of marble, would the reaction be faster or slower ? 4. On your graph – in a different colour- draw on the line that you think a lump of marble would produce. Adapted from LCW Booklets 51 Investigating particle size: Indigestion Tablets Learning intention Describe how particle size affects the action of indigestion tablets. Read Stomach acid is really hydrochloric acid. Its pH number is about 2. You learned in S2 that indigestion can be caused by too much acid being made in your stomach. This causes pain. The acid can be 'cancelled out' or as chemists called it "neutralised" by taking an 'antacid' tablet. When the acid is being neutralised, its pH number rises towards 7. We used the pH Sensormeter and a computer to compare how fast the neutralisation was taking place using whole tablets and powdered tablets. Here is how it was set up: Adapted from LCW Booklets 52 Success task Now collect a graph of the results and discuss the findings with your partner and write a conclusion for these results in your jotter. Adapted from LCW Booklets 53 Temperature and the speed of reactions Learning intention Describe how temperature affects the rate of a reaction. Read The temperature of chemicals can affect how fast they will react together. Watch your teacher demonstrate how to do the next experiment. measuring cylinder beaker Hydrochloric acid Sodium thiosulphate Paper with X drawn on it The time for the reaction can be measured by timing how long it takes for the X to disappear. Again the data logger is being used to follow the reaction and produce a graph) 1. What was the aim of the experiment (what were you trying to find out)? 2. How did you measure the time for the reaction? 3. Draw a diagram of the experiment. 4. How did you change the temperatures? 5. Design a table for your results with two headings. Adapted from LCW Booklets 54 6. What is your conclusion (what did you find out)? 7. Make a list of at three things you did to make sure that the three experiments you did were fair. 8. Looking at the results on the graph, which reaction was faster? Explain your answer. Copy and complete the sentence below. The ________ the temperature the __________ the reaction. Discuss the following questions with your partner. 1. Where would food rot faster - in a fridge or in a freezer? Explain your answer. 2. Where do plants grow faster - in a greenhouse or outside? Explain your answer. 3. Sarah carried out an experiment to compare the speed of the reaction of a metal with some acid. Her results are shown in the table below. Temperature(C) Time for all of the metal to be used up (sec) 0 78 20 45 40 23 60 10 a) Copy the table of results into your jotter. b) Copy and complete the sentences below: Adapted from LCW Booklets 55 As the temperature increases, the time for the metal to be used up _________________. As the temperature increases the reaction gets ____________________. Adapted from LCW Booklets 56 Concentration and the speed of reactions Learning intention Describe how changing the concentration changes the rate of a reaction. Read The concentration of a solution can change the speed of a reaction. Remember back to the Solution topic you completed recently. We found out that the unit of concentration of a solution in chemistry is moles per litre which is sometimes shortened to mol/l. Question: Which solution has the higher concentration; 0.1 mol/l or 1.0 mol/l? PPA Experiment: Rates of Reaction Experiment: Effect of concentration Success task PPA experiment completed accurately. Adapted from LCW Booklets 57 Catalysts Learning intention Describe the effect that a catalyst has on the rate of a chemical reaction. Read Another way to speed up a chemical reaction is to use a chemical called a catalyst. A catalyst speeds up a reaction but is not used up so it can be used over and over again. Your teacher may demonstrate (demo 1.29) a reaction where a catalyst can be used to speed up a reaction to make oxygen. Glowing splint Hydrogen peroxide Adapted from LCW Booklets Glowing splint Hydrogen peroxide + catalyst 58 Write a heading and answer the following questions. 1. What is the name of a chemical that can be used to speed up a chemical reaction? 2. Why is it possible to use a catalyst more than once? 3. Copy a diagram of the demonstration that you saw and add bubbles where you say them. 4. What happened to the glowing splint in the experiment without the catalyst? 5. What happened to the glowing splint in the experiment with the catalyst? 6. What does this tell you about the name of the gas that is made in the reaction? 7. Which reaction made the gas the fastest? 8. Your teacher used 1g of catalyst in the second experiment. a) How much catalyst would there be after all of the solution had reacted? b) How could you separate the catalyst from the solution to use it again? 9. Copy the sentences below. Adapted from LCW Booklets 59 Use of catalysts Catalysts are often used in motor car exhaust systems. The part of the exhaust that contains the catalyst is called a catalytic convertor. The catalytic convertor changes harmful and poisonous gases into safer ones that can go out into the air. Success task Draw a poster that shows how a chemical reaction can be speeded up – use information from the multi media science schools programme and your experimental conclusions. Adapted from LCW Booklets 60