chemistry 36 - Penn State PHP Service - Home
advertisement

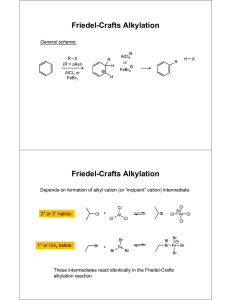
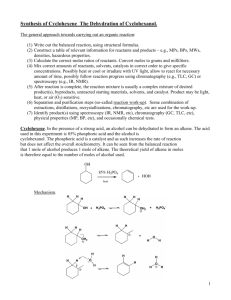
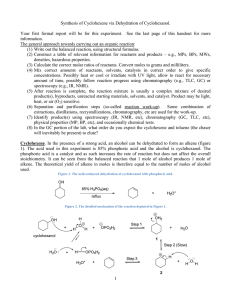
CHEMISTRY 36 SPRING 2006 Dr. John Tierney REQUIREMENTS - TEXT: ORGANIC CHEMISTRY LABORATORY MANUAL , 2nd Edition by Svornos, Sarlo & Kulawiec; published by William C. Brown, Dubuque, Iowa. You must turn off all cell phones and pagers. Cell phones and pagers are not allowed to be used in the lab at any time. LAB NOTE BOOK: Supplied by the Instructor. COURSE DEMANDS: This is a practical course in chemistry. You will be employing the theory and knowledge that you have gained from CHEMS 38 and 39 in a laboratory setting. It is always important that you relate this theoretical knowledge to operations that you are carrying out in the laboratory. Before proceeding with any part of an experiment, particularly in preparations, always ask yourself... "what are the implications of carrying out this process". This approach will minimize the possibility of an accident through the addition of the wrong solution or substance. For this reason IF ANY STUDENT IS LATE FOR CLASS AND A DISCUSSION OF THE EXPERIMENT HAS STARTED YOU WILL NOT BE ALLOWED TO COMPLETE THE EXPERIMENT; THIS WILL HAVE A DETRIMENTAL EFFECT ON YOUR GRADE READ ATTACHED PHILADELPHIA INQUIRER ARTICLE DATED 1/9/95. SAFETY (PAGES 1-2 in text): SAFETY GLASSES ARE TO BE WORN AT ALL TIMES WHILE IN THE LABORATORY. You should become acquainted with the locations of the fire extinguishers, fire blankets, eye bath, shower and nearest exit in case of an accident or fire. Anyone not wearing safety glasses will be asked to leave the laboratory, repeat offenders stand the possibility of failing the course for improper laboratory procedure. There will be NO EATING OR DRINKING in the laboratory. In fact, it is advisable that you refrain from bringing any food or beverages into the laboratory even with the intention of later consumption. There is always the potential for contamination by chemicals. Always ensure that you thoroughly wash your hands before leaving the laboratory, particularly if your next destination is the cafeteria. Neoprene gloves are provided for your use and it is advisable that you wear these whenever carrying out an operation using chemicals. Any student who squirts water or behaves in any other irresponsible manner in the laboratory will be asked to leave immediately and will receive an F grade for the course. GRADING: (1) Lab Note Book – All your experimental details must be recorded in the lab notebook that is provided. Nothing should be recorded on scraps of paper. The data collected in the lab notebook will be formally written up in a lab report. All experiments will be graded out of 100 points. You will receive points for correct set-up (during lab) product appearance, yield (weight and % yield) and accuracy of your laboratory write-up. The lab report write-up format is on the attached sheet. The lab notebooks will be looked at on a weekly basis and points will be deducted from the report grades if the notebook is not being used appropriately. LAB REPORTS The lab reports will be prepared from the data collected in the lab notebook. The lab report will be due the lab following each lab class, unless otherwise noted. The report will be typed as a Word (.doc or .rtf file). All chemical structures and equations will be drawn using Isis Draw free software. PREPARATIONS FROM BOOK OR PROJECT: After the unknown compounds have been analyzed and identified, each student will have one of two options. Either they will continue with selected preparations from the textbook, or they will embark on a mini-research project to the end of the semester. The direction taken will be at the discretion of the student in consultation with the instructor. Projects will be done on a small group basis with collaboration between students. ACADEMIC INTEGRITY: Cheating on a single lab report will result in a zero for that lab report. Attempts to turn in someone else’s product as your own product because of a problem with a lab will result in a zero for that lab. Repeated instances of cheating will result in a grade of ‘F’ for the course. (PSU Senate Policy 49-20) PLAGIARISM: In a situation where another person’s published work is handed in as though it is work that you have done, you will receive zero credit for that assignment. Repeated counts of plagiarism will result in a grade of ‘F’ for the course. Other author’s published work must be given the correct recognition, by reference, when handed in with related work that you have completed. (PSU Senate Policy 49-20) STUDENTS WITH DISABILITIES: Penn State University does not discriminate against qualified students with documented disabilities in its educational programs. If you have a learning disability-related need for modifications in the course, or if you have a physical disability-related need for modifications in the course, contact Sharon Manco, 610 892 1461, 203A Main. This notification should occur by the end of the first week of the semester. --------------------------------------------------------------------------------------------------------------------I, the undersigned, have carefully read and understood the above instructions. I have paid particular attention to the information given under the section heading entitled "SAFETY". ____________________________ signature ______________________________ name – printed _________ date DATE* TOPIC 1/09 CLASSES BEGIN DROP ADD BEGINS (01/09) LATE REGISTRATION BEGINS (01/09) 1/10 1/12 Check-In and procedures Melting points (pp 19-23) and boiling points (pp 25-28) 1/16 1/17 1/19 Martin Luther King Day Simple and Fractional Distillation (pp 29-35) Steam Distillation (pp 37-41) 1/24 1/26 Steam distillation continued if necessary Liquid-liquid extraction (pp 54-61) 1/31 2/02 Crystallization-recrystallization (pp 63-77) Column chromatography (pp 42-45) 2/07 2/09 Thin Layer Chromatography (pp 45-49) Equilibrium constant (pp 78-81) 2/14 2/16 Synthesis of cyclohexene (pp 123-126) Analysis of cyclohexene 2/21 2/23 Friedel-Crafts Alkylation (pp 192-194) Friedel-Crafts alkylation continued 2/28 3/02 Solvolysis of t-butyl chloride (pp 154-157) Nitration of bromobenzene (pp 188-191) 3/06 - 3/10 Aldol condensation (pp 243-246) Finish aldol condensation 3/21 3/23 Identification of an unknown (pp 83-113 & 325-336) Solid unknown (Start project discussions) 3/28 3/30 Solid unknown continued Liquid unknown 4/04 Start Research Project 4/06 Research Project If unknowns have been identified LATE DROP ENDS (04/07) Research Project – EURECA PRESENTION (04/20) 4/27 Check-out 4/28 Last Day of Classes and Last Day to Withdraw NO CLASSES Work with a partner, one does simple distillation, the other fractional. DROP ADD/ENDS (01/18) LATE REGISTRATION ENDS (01/18. $6 SCHEDULE ADJUSTMENT FEE BEGINS (01/19). SPRING BREAK 3/14 3/16 4/11 - 4/27 NOTE Note that these dates are tentative and are subject to change. We may overlap some labs and some days may be lost to class cancellation due to snow