Title: Potato & Carrot Lab: Investigating Osmosis and Diffusion
advertisement

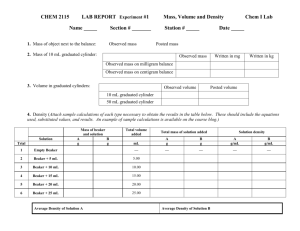
Title: Vegetable Osmoregulation Investigation Problem: How are vegetable samples affected by hypertonic solutions? How are vegetable samples affected by hypotonic solutions? Hypothesis (Prediction): If IV then DV because… Experimental Design Independent Variable (IV): Levels of IV: Dependent Variable (DV): How will the DV be measured and/or observed? Controlled Variables (2+): Experimental Control Group: How will you set up the beaker with only experimental controls? Which label will it have? Experimental Group: How will you set up the beaker that will answer the investigative question? Repeated Trials: Materials: 2 small beakers 2 pieces of carrot 2 pieces of potato Salt Balance & weighing paper Scoopula to help mass salt and to mix the saline solution Toothpicks to suspend the vegetable samples in the water 2 pieces of string for carrot turgor pressure Procedures: 1. Your entire table will be your lab group. Devise a plan for working as a team, and then gather materials. 2. Label two beakers A and B with your lab group name clearly marked on both. a. Beaker A: Add 100 mL of tap water b. Beaker B: Add 100 mL of tap water and 10 grams of salt. Mix solution until all the salt is completely dissolved. 3. Snuggly tie a 20-30cm piece of string around each piece of carrot. Be sure to note which carrot will be placed in which beaker. 4. Record the mass (in grams) and length (in mm.) of the 2 potato and 2 carrot samples (with string) of equal size and record the mass and length on the data table for the appropriate beaker, A or B. 5. Place one carrot and potato sample on the toothpick in such a way that the samples are suspended and submerged in the two beakers. 6. Sketch the two beakers, after the specimens have been suspended in them. Be sure to date the sketch. 7. Store your beakers as instructed and thoroughly clean up your work area. 8. Check the specimens in 24 hours, and then again the next class period, and record your observations. 9. Devise a plan for working as a team, and then gather materials. 10. Label two beakers A and B with your lab group name clearly marked on both. a. Beaker A: Add 100 mL of tap water b. Beaker B: Add 100 mL of tap water and 10 grams of salt. Mix solution until all the salt is completely dissolved. 11. Snuggly tie a 20-30cm piece of string around each piece of carrot. Be sure to note which carrot will be placed in which beaker. 12. Record the mass (in grams) and length (in mm.) of the 2 potato and 2 carrot samples (with string) of equal size and record the mass and length on the data table for the appropriate beaker, A or B. 13. Place one carrot and potato sample on the toothpick in such a way that the samples are suspended and submerged in the two beakers. 14. Sketch the two beakers, after the specimens have been suspended in them. Be sure to date the sketch. 15. Store your beakers as instructed and thoroughly clean up your work area. 16. Check the specimens in 24 hours, and then again the next class period, and record your observations. Data Analysis:. After the data table is completed, construct a line graph that shows your experimental results. You will need to construct 2 different line graphs, one for mass and one for length. Graph Potato mass and length and Carrot mass and length. Conclusion: You will write a CEC formatted conclusion in your Journal Claim: Restate your hypothesis and indicate whether it was supported, not supported or contradicted? Evidence Cite specific data points from both conditions on both vegetable samples in your explanation. High values compared to low in which condition. Commentary Explain why your hypothesis was supported or not. Did the vegetable samples change as you predict for color, texture, smell, tightness of the string on the carrot, etc. What was the purpose of tying string around the carrot? Which condition did it become looser, which condition did it become tighter and why? An increase in potato or carrot mass and length indicates that which process happened to the vegetables? Which solution was most effective at increasing mass and/ or length? What would the individual cells look like in each solution? Draw a labeled diagram for each that shows the direction the water moved in the cell. Why do they spray the veggies down in the produce aisle, etc. Data & Observations: Quantitative Observations Initial Beaker A 24 hours Beaker A 24 hours Beaker B 48 hours Beaker A 48 hours Beaker B Qualitative Observation Beaker Sketches Beaker A Beaker A Beaker A Beaker B Initial Beaker B Date: Beaker B Date: Beaker B Date: