Lab 4 - Osmosis
advertisement

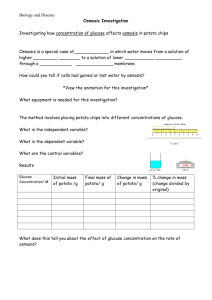
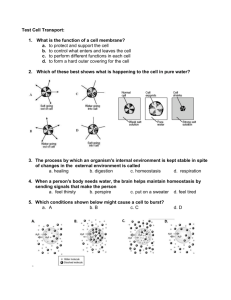
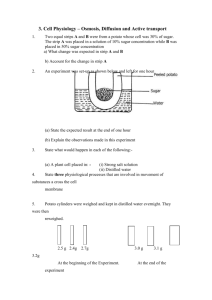
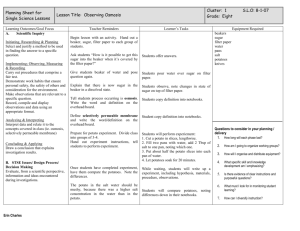
Lab 4 – The Puffy Potato: Osmosis in Cells (Note: Your completed lab report should include title, problem, pre-lab questions, data table, answers to questions, and conclusion.) Problem: Do potatoes stay crisper in fresh or salt water? Background Information: Remember that all cells are surrounded by a cell membrane. The membrane allows for the exchange of materials with the environment. Materials needed by the cell, such as water and food are allowed in; while wastes, such as carbon dioxide and other products of cellular metabolism are allowed to leave. To understand how materials move into and out of the cell, you must understand the process of diffusion. All materials including water are made of particles called atoms and molecules. These particles naturally travel from areas where they are crowded, to areas less crowded where they can spread out. Diffusion is the movement of these particles from an area of high concentration to an area of lower concentration. This movement can occur across cell membranes, both into or out of the cell. Cells do not need to use energy for this to occur because it happens naturally, so this is called passive transport. Water is important to cells because it makes up so much of the cell’s matter (over 70%), and because it is “the universal solvent” that dissolves and carries many of the other chemicals the cell needs. The diffusion of water then is so important that it has been given its own name osmosis. Osmosis is defined as the diffusion of water across a cell membrane. When the cell needs to move particles in or out and must move them against this normal flow (that is, when the cell needs to get something from an area of low concentration to an area of an already high concentration), it must use some energy to do so (some ATP). When this happens it is called active transport because it must actively use energy to do the work. Book Reference: pages 84-87 (Light Green version, pages 90 – 92) Pre-lab Questions: (Read background information and procedure before answering.) 1. In the example below, which statement would be most correct? Explain your answer. X X X X Outside of the Cell X X X X X X X X X X X X A. B. C. D. X X X X Inside of the Cell X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X Molecule X will move into the cell by osmosis. Molecule X will move into the cell by passive transport Molecule X will move out of the cell by osmosis. Molecule X will move out of the cell only with an input of energy. How does a plant cell get “crisp”? What part of the plant cell would store liquids? How does a plant “wilt”? What are we measuring to determine crispness? 2. 3. 4. 5. Materials: (per group) Potato slice Two cups Bottle cap Distilled Water Safety Goggles Salt Water Digital Scale Paper towels Procedures: 1. Create a data table. 2. Cut out two pieces of potato slice using the bottle cap, measure the mass (weigh), and record the data. 3. Put each piece into a properly labeled cup: (1) for salt and (2) fresh water. 4. Leave them for 30 minutes and work on another assignment. 5. After 30 minutes, take each piece out of the water, measure the mass, and record the data 6. Try to put your pieces back into the slice to see if they “fit”; record your observation. 7. Throw out the potato pieces and water solutions, clean up all other materials and put them away. Safety Considerations: Wear goggles Be very careful not to mix up your samples when weighing and putting into respective solutions. Be cautious with spillage and make sure you wipe off the balance. Leave all materials as you found them! Post-Lab Questions: 1. What happened to the weight of each slice? Why? 2. What does the weight change in the slices have to do with osmosis? 3. If you worked in a restaurant, what would you do to keep potatoes fresh, based on the findings of this lab? Conclusion: (In your conclusion, you should briefly summarize the problem and what you did. Then answer the problem referring to the data and background information including your pre and post lab questions). Don’t forget to tie the results back to the background information on osmosis and passive transport! This is the key to getting a good grade. Links: Good Introduction – http://www.purchon.com/biology/osmosis.htm Good Visual – www.bbc.co.uk/education/asguru/biology/01cellbiology/05pathways/10osmosis/index.shtml