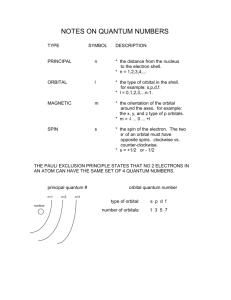
Odd number electron system. In a closed shell molecule with 2
advertisement

Odd number electron system.
In a closed shell molecule with 2 electrons in every molecular orbital there
are clearly as many α spin electrons as there are β spin electrons and the spinorbital energy
k
X
a = Hac +
(Jas − Kas )
s=a
is the same for both the electrons in a molecular orbital. The results of a HartreeFock calculation on a closed shell molecule include the molecular orbital energies
with out reference to spin.
In an odd number electron molecule, the unpaired electron is taken to have
α spin and clearly every α spin has one more exchange interaction than does
its β spin partner. Two electrons formally occupying the same orbital actually
have different energies. The results of a Hartree-Fock calculation on an unpaired
electron system include a spin orbital energies - a separate list of energies for α
and β spin orbitals.
The difference in energies between pairs of electrons technically in the same
molecular orbitals depends most critically on the orbital energies. For low energy
orbitals it is very small, for orbitals close to the HOMO it can be of the same
order as the separation of molecular orbitals. The most extreme case is the
unpaired electron itself as the α spin-orbital is the HOMO and its β partner is
a virtual orbital, generally at about the same energy as the, α, LUMO.
Differences in energy are only possible if there is a difference in distribution.
The two spin-orbitals arising from the same molecular orbital have different spatial distributions. In fact the usual concept of a molecular orbital is somewhat
lost and is replaced by a molecular spin orbital. (Most chemists are blissfully
unaware of this important distinction and happily draw orbital diagrams with
pairs of electrons in molecular orbitals even when there is an unpaired electron
in the molecule.)
The Hartree-Fock theory of unpaired electron systems requires two sets of
equations,l one for α and one for β spin orbitals. The expression for the Fock integrals Fmn are necessarily more complicates and the equivalent of the RoothenHall equations are called the Pople-Nesbit equations.
Evidence that the Hartree-Fock theory is approximate.
This section returns to the essential point of all the earlier sections, namely
that the Schrödinger Equation is insoluble and therefore the Hartree-Fock theory
must be an approximation. This should result in the theory making predictions
that are not possible or at least not observed.
Consider the bonding molecular orbital of the hydrogen molecule, Ha Hb ,
using a minimal basis set.
ψ = N (1sa + 1sb )
the SDW formed from this is:
Ψ(1, 2) = |ψα ψβ| = (1/2)1/2 {ψ(1)α(1)ψ(2)β(2) − ψ(2)α(2)ψ(1)β(1)}
1
substituting for ψ and expanding gives:
= |1sa α 1sa β| + |1sb α 1sb β| + |1sa α 1sb β| + |1sa β 1sa α|
+
+
the first two terms corresponds ionic forms of the molecule, H−
1 – H2 and H1 –
(both the electrons on one or other of the atoms) and the second two terms
correspond to covalent forms of the molecule, H1 – H2 . The expression for the
LCAO of the molecular orbital is the same for all values of the bond length R,
the value of N changes with R but not the LCAO part. For dissociation of the
molecule - R very large - the orbital predicts that there is an equal probability of
the dissociation products being ions or atoms. In reality dissociation to atoms
is the only process that occurs! Hartee-Fock theory predicts the wrong products
of the dissociation reaction. In fact Hartee-Fock theory makes bad predictions
in all molecular problems involving long distances; dissociation, non-classical
bonding; transition states; etc ...
The reason is the definition of the Jr (i) and Kr (i) parts of the Fock operator
F (i). In reality the two electrons in the hydrogen molecule will be as far apart
as possible to minimize their repulsion. Their motions are correlated.The
definition of the Jr (i) and Kr (i) averages the position of the electrons and looses
this correlation of their positions. It allows them to be too close together. The
Hartee-Fock energy is too large.
H−
2
Unrestricted Hartree-Fock calculations.
If the Hatree-Fock calculation on the hydrogen molecule is performed using
the Pople-Nesbit equations the α and β spin orbitals of the same molecular orbital are allowed to have different spatial distributions. This allows one electron
with α spin to build up on one atom whilst the other builds up on the other
atom. It results in predicting the proper products of dissociation.
Using Pople-Nesbit for closed shell molecules is called Unrestricted HartreeFock (UHF) as opposed to the Roothan-Hall equations which is restricted HatreeFock (RHF). At the minimum energy geometry of the molecule, RHF and UHF
give identical results. UHF does not solve the problem of the lack of correlation
of the motion of the electrons but it does improve the long bondlength results.
(Diagramatic description in lectures.)
Post Hartree-Fock Calculations
One possible way of achieving a better result than UHF would be to allow
the SDW to be manipulated in some manner. For example, if the wavefunction
were written:
Ψ(1, 2) = (1 − µ){|1sa α 1sa β| + |1sb α 1sb β|} + µ{|1sa α 1sb β| + |1sa β 1sa α|}
that is:
Ψ(1, 2) = (1 − µ)(ionic) + µ(covalent)
then µ could be used as a Variational Parameter and optimized to obtain the
lowest energy. In fact such a calculation is possible for the simple case of hydrogen molecule and a value of µ ∼ 0.8 is found to be optimal.
This approach is not practical for larger molecules.
2