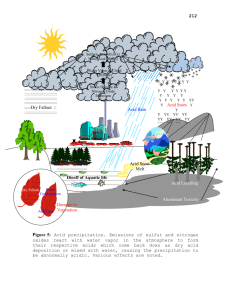
Chapter 15 text
advertisement

15-1 Chapter 15 Climate Fluctuations and Global Change Have you ever been in a building where one room feels much colder, or warmer, than the others? To explain why this room is colder than the others are we would investigate the differences in the energy gains and losses of the rooms. The energy budget of a room depends on many things including how many windows it has, the direction the windows are facing, the floors and walls, and the heating and ventilation systems. The number of windows and the direction they face play a role in how much sunshine enters the room. Heat losses out windows can be large depending on how well the window frame is insulated and whether the windows are single or multiple paned. The condition of the walls and floor also play an important aspect of the heat budget of the room. If the walls are poorly insulated they will be cold and, even if the air temperature is 70F, the room will feel cold because of the energy imbalances felt by the human body. The air circulation will also play a role in the climate of the room. A strong blower, like a fan, would circulate air, and heat, in the room, while poor circulation may not spread heated air evenly throughout the room. These factors all related to energy exchanges, determine the climate of a room. Earth's climate is also determined by the circulation patterns of the air and how heat is transferred. Climate is controlled by complex interactions among the individual components of the Earth/sun system. The basic parameters that change our climate are solar energy input, the state of the atmosphere, the hydrological cycle and the biosphere, including human activities. It is the general opinion of the scientific community that a global 15-2 climate change will occur in your lifetime. This chapter uses specific examples to explore the underlying causes of regional and global climate change. 15-3 Observations of Global Warming Weather conditions change both rapidly and slowly. In less than an hour, a thunderstorm can change a bright sunny day into a dark rainy one. Temperature and precipitation also vary from one year to the next. There is convincing evidence that climate varies naturally on time scales from interannual variations to changes that occur over millions of years. Over the last decade, the global average temperature has increased (Figure 15.1). Is this change a natural fluctuation in our climate, or is it a result of human activities in the last hundred and fifty years or so? Natural variations in climate make it difficult to distinguish long-term trends caused by humans. While a few warm winters and hot summers do not mean global warming, the observed warming trend over the last two decades is indicative of a global change. This trend in global warming has resulted in debate about what measures might be taken to mitigate this warming. Most of these measures involve changing human activities, such as reducing the burning of fossil fuels and deforestation. Humans currently burn about 6 billion tons of fossil carbon per year, with the rate of burning increasing about 2 percent per year. The oceans and forests absorb only about half the carbon dioxide emitted into the atmosphere. Because of the potential impact on society, global warming is frequent topic in news reports. Sometimes these reports suggest that scientists are arguing whether greenhouse gases will change the climate. In most scientific discussions, the issue is not whether greenhouse gases will induce a climate change. The issue is what the effects will be and how these can be detected best. There are several factors affecting our climate. These factors combine to cause fluctuations on many different time scales (Table 15.1). Factors that cause long-term 15-4 fluctuations in climate include changes in the sun’s output of energy, the Earth's orbit about the sun, and changes in ocean/atmospheric circulation. Fluctuations on a shorter time scale can be caused by changes in clouds and water vapor, and increased concentration of greenhouse gases due to human activities. To address the issue of global warming, this chapter provides examples of climate fluctuations caused by natural phenomena as well as those due to human activities. The chapter also provides a foundation to answer questions that will arise about climate change in the future. Table 15.1 Cause of Climate fluctuations and approximate time lines. Cause of Climate Change Number of Years Human effects on land surface 1-100 Human effects on atmosphere 1-100 Volcanism 1-1000 Solar variability 10-1000 Air-sea interaction 1-100,000 Orbital variations 10,000-100,000 Plate Tectonics 100,000-100,000,000 Earth's orbit Solar radiation is the source of energy that drives the dynamics of the Earth’s atmosphere and oceans, therefore its climates. Changes in the output of the sun's radiation affect Earth's climate (Box 15.1). Any change in a planet's orbit about the sun 15-5 can also lead to a climate change. Variations in Earth's orbital movements around the sun generate very slow climate cycles. Called the Milankovitch cycle, these variations affect the amount of solar energy the Earth receives and where on the planet that solar energy is absorbed. Changes in this energy distribution affect climate. The Milankovitch Theory proposed by Milutin Milankovitch in the 1930s, attributes climate change to natural variation in the sun-earth astronomical relationships. There are three independent cycles, which combine to produce variations in Earth's orbit around the sun and consequently affect the distribution of solar energy on the planet. These cycles are referred to as the eccentricity, obliquity, and precession of the Earth's orbit. The earth orbital eccentricity describes changes in the shape of the earth's orbit around the sun. Figure 15.2 shows that Earth's orbit around the sun varies from a near circular orbit to an ellipse. The more circular the orbit, the more uniform the sun's rays on Earth, because the variations in distance from the sun are smaller. An increase in the eccentricity results in an increase in insolation in the summer hemisphere and a decrease in the winter hemisphere. This tends to amplify the season cycle in the high and midlatitude regions. To go from a near circular orbit to the most extreme ellipsoid takes about 100,000 years. Currently, the orbital changes that are occurring are making the Earth's orbit more elliptical. Changes in Earth's tilt angle, or obliquity, (Figure 15.3) determines how different the seasons will be in a given hemisphere. The angle of inclination of Earth (see Chapter 2) is presently 23.5 off the perpendicular. The larger the angle the greater the differences in the seasonal weather of middle and high latitude locations. Summers will be hotter 15-6 and winters colder when the axis of tilt is large. Over a 41,000 year period this angle varies between 22 and 24.5. The Earth's current tilt is about mid-way between these extremes. The third orbital parameter describes the Earth's wobble as it revolves around the sun. If you spin a top, you will notice that it wobbles in the sense that the spin axis rotates with time (Figure 15.4). The same happens with Earth. The wobble is known as the precession of Earth's axis. Because of this wobbling, the time when the earth is closest to the sun advances by about 025' a year, or a period of 27,000 years. Today, Earth's axis of rotation is pointed towards Polaris, also known as the North Star. Because of precession, 13,500 years from know it will be pointing toward Vega. The precession is a very slow motion that determines the time of the equinoxes (Figure 15.5). The precession and the time of aphelion and perihelion define the differences between winter and summer condition, or the seasonality. Warmer summers and colder winters will result in the Northern Hemisphere when the axis of rotation is pointed towards the sun during perihelion. In this case, the seasonality of the Southern Hemisphere would be decreased. These cycles are the main factor behind the onset and retreat of the ice ages. Major ice sheets occur every 100,000 years. Superimposed on this are smaller ice advances every 41,000 and 23,000 years. These periods coincide well with the natural variations of Earth's orbit and orientation to the sun (Figure 15.6). While the Milankovitch cycles may initiate global climate change, these cycles alone cannot fully account for the observed variations in temperatures. This 10% variation in the solar energy gains due to variations in Earth's orbit is not enough to explain the 10C (18F) observed swings in temperature. The conclusion is that orbital changes are not the only 15-7 forces responsible of the ice ages. An additional factor maybe changes in CO2 concentration. Analysis of air trapped in bubbles in ice sheets shows that CO2 levels are lower during colder glacial periods than during interglacial periods. These changes in CO2 levels are likely due to changes in biological activity and would enhance the changes caused by orbital and positional variations. These additional effects are considered feedback mechanisms. Feedback Mechanisms A positive feedback mechanism is one that enhances an existing trend of a change in climate. It is through positive feedback mechanisms that small Feedbacks occur when one change leads to some other change, which can act to either reinforce (positive feedback) or inhibit (negative feedback), the original change. changes can lead to large ones. For example, the amount of water vapor in the atmosphere is a positive feedback mechanism involved in climate change. As the air temperature warms there is increased evaporation from surface waters, resulting in higher atmospheric water content. Water vapor is a greenhouse gas. So, the atmosphere warms even further, causing more water vapor to evaporate into the atmosphere, enhancing the warming, and so on. While human emissions of CO2 are attributed to the current trends in global warming, it is the water vapor feedback that causes most of the warming. Another example of a positive feedback mechanism is the ice-albedo temperature feedback. Ice sheets have a high albedo and affect climate by reflecting more sunlight than other types of surfaces, such as bare ground and areas covered by vegetation. This reduces the amount of sun's energy that can warm the planet’s surface. All things being equal, the more ice the cooler the Earth. Ice-covered areas could expand when the 15-8 atmosphere along the margins of established ice regions cool. The increased area of ice reflects even more sunlight and further reduces the amount of solar energy absorbed by the surface. This reduction in solar energy gains causes a further cooling and results in the formation of more ice, and so on. This is a positive feedback loop; more ice causes a cooling which reduces the temperature so that more ice develops, leading to a further cooling (Figure 15.7). Correspondingly, a retreat of an ice sheet is also a positive feedback since it would cause a warming, due to the lower albedo, and lead to a warming and a further retreat of the ice sheet. A negative feedback mechanism mitigates an existing trend of climate change. A simple example of a negative feedback mechanism involves the influence of CO2 concentration on plant photosynthetic rates. In the process of photosynthesis plants use carbon dioxide and water to make sugar. An environment rich in CO2 accelerates the growth of many plant species. This is a negative feedback: increasing CO2 concentrations allow plants to grow faster and thereby increase the overall photosynthetic rate which removes increased amounts of CO2 from the atmosphere. So, by a negative feedback loop more carbon dioxide results in its decrease. Of course, there are other limiting factors in a plant's ability to respond to an enriched CO2 environment, such as a lack of water nutrients. Also, insects and pests might also enjoy the warmer environment associated with high concentrations of CO2 which can reduce the number of plants. Feedback processes can quickly become complicated even in the simplest climate change scenario! In many of the discussions that follow, we will focus on primary processes that can lead to a change in climate. We will limit discussion of secondary processes 15-9 associated with positive or negative feedback. These primary processes include changes in amounts and concentrations of aerosols, greenhouse gases, ocean circulation patterns, and surface properties. Aerosols As you learned in Chapter 1, aerosols are small solid particles and liquid droplets (excluding cloud droplets and precipitation.) Aerosols are also called particulates. Aerosols typically range in size from 0.1 to 100 microns. The period at the end of this sentence is about 10 microns in diameter. Natural processes, such as volcanic eruptions or anthropogenic processes (human activities), such as automobile exhausts, form aerosols. There are primary and secondary sources of aerosols. Primary sources are those processes that directly emit aerosols into the atmosphere. Examples are dust storms, automobile emissions, volcanic eruptions, and smoke from agricultural fires. In the case of secondary sources, aerosols form as a result of chemical reactions in the atmosphere. An example are biogenic aerosols that form from sulfur-bearing gases, primarily dimethyl sulfide (DMS), generated by ocean biology. Most aerosols are generated by natural process (Figure 15.8). Aerosols sometimes have notable effects on the condition of the atmosphere. Large concentrations can be extremely hazardous. In large concentrations, such as in dust storms, (Figure 15.9) dust presents a health hazard, suffocates livestock, and severely limits visibility causing hazardous traffic conditions. Heavy air pollution poses a health hazard. An extreme case of this occurred on December 5-9, 1953 when an estimated 3500 to 4000 people died in London, England. Over this five day period Smog originally a combination of smoke and fog, this term is now used to describe mixtures of pollutants in the atmosphere. 15-10 stagnant moist air combined with the smoke from burning of low-quality coal to produce a lethal mixture of fog and smoke. This combination of smoke and fog produced a pollution event called smog. Today, smog is a generic term used to indicate polluted air (Box 15.2). Increases in aerosol concentration can occur naturally, as in volcanic eruptions, or due to human activities, as in the manufacture of chemicals and particles from burning fossil fuels. Regardless of how aerosols enter the atmosphere they can modify the energy balance of a region and affect climate by changing the radiation budget of the planet. The general climatic impact of most aerosols is to reduce the solar radiation reaching the surface by scattering radiation out to space. The type of aerosol determines the degree to which it impacts climate. Volcanic Eruptions Chapter 3 introduced the concept of how volcanic eruptions can affect climate. Debris from Mt. Tambora (8S latitude, 118E longitude) (Figure 15.10) resulted in the year without a summer. Large quantities of ash, dust, and sulfur dioxide can be injected into the stratosphere during a violent eruption. Since the stratosphere is stable due to the increase in temperature with altitude, volcanic debris stays in the stratosphere for a couple of years. The long residence time of volcanic debris modifies the energy balance of the planet. The amount of cooling due to a volcanic eruption is determined by: The force of the eruption. For a global impact, the debris from the volcanoes must be injected into the stratosphere where it can remain for many months. A large, forceful eruption can put dust into the atmosphere. 15-11 The amount of sulfur dioxide (SO2) in the volcanic plume. In the stratosphere, SO2 combines with water vapor to make tiny particles of sulfuric acid. The particles reflect solar radiation back to space, reducing the amount of solar energy at the surface. Latitude and winds in the stratosphere. Stratospheric winds at the time of the eruption and the latitude of the volcano determine how the volcanic plume will spread. For global affect on weather, the sulfuric acid particles have to spread over the globe. Not all volcanic eruptions result in a cooling of the earth. Here we will compare three recent volcanic eruptions: Mt. St. Helens, El Chichón, and Mt. Pinatubo. The eruption of Mt. St. Helens in the state of Washington in May of 1980 produced little, if any, effect on global temperatures. While a violent eruption, most of the force of the eruption was horizontal, resulting in relatively little debris being injected into the stratosphere. The emissions from Mt. St. Helens primarily stayed in the troposphere and the ash and dust settled quickly to the ground. El Chichón, Mexico erupted in 1982. El Chichón was a much less violent eruption than Mt. St. Helens; however, debris was injected into the stratosphere. The global spread of El Chichón’s stratospheric debris was primarily limited to between 5 N and 40 N latitude. Estimates of the global cooling due to the eruption of El Chichón are approximately 0.3 to 0.5 C. El Chichón also had much more sulfur dioxide in its plume than Mount St. Helens. The cooling effects of the El Chichón eruption may have also been hidden by the warming caused by El Niño (Chapter 8). Mt. Pinatubo was this century’s most violent eruption (Figure 15.11), injecting over 25 million tons of sulfur dioxide into the stratosphere. Once in the stratosphere, tiny 15-12 sulfuric acid particles formed and rapidly spread all over the globe. These tiny particles reflected solar energy back to space and resulted in a cooling of the globally averaged surface temperature by approximately 0.6C (1F). The NASA Earth Radiation Budget Experiment program measured the effect of the Mt. Pinatubo’s stratospheric aerosol on the radiation balance of the planet and for the first time provided firm scientific evidence that volcanic eruptions cool the Earth. Figure 15.12 shows the departure of the average global air temperature between 1970 and 2000) from the 1951-1980 average temperature. Mount Pinatubo erupted in June 1991. This provided a unique opportunity to evaluate long term weather predictions by putting the aerosols into the models and predicting the global mean temperature. The weather models predicted that after about one year, the average hemispheric temperatures should decrease by about .2 to 0.5 C. By July 1992 the global mean temperature had decreased by approximately 0.5 C. During this time there was also an El Niño event that lasted from 1990 and 1995, so the cooling due to Mt Pinatubo might even have been greater, where it not for the warming associated with El Niño. Aerosols generated by human activities The atmosphere is a complex mixture of gases and aerosols. Human activities modify the air we breathe (Box 15.2) and the climate we live in. The atmospheric concentration of sulfur dioxide has increased over the last century due to human-related activities. Sulfur dioxide (SO2) is an industrial by-product that arises from the burning of sulfur. The major source of SO2 from human activities is the burning of coal that contains sulfur. SO2 can be a hazard to health. SO2 is important for climate change as it 15-13 can produce aerosols that scatter solar radiation back to space, causing a cooling of the planet. Acid Deposition Air pollution from industrial areas can become acidic and carried downwind for many Acid Deposition refers to the falling of acids and acid-forming compounds from the atmosphere to Earth's surface. miles. When these acids settle on the ground, they can damage plants and aquatic life. This settling may occur as dry particles (dry deposition) or as rain, snow, or fog (wet deposition). When in the form of rain, the acid deposition is referred to as Acid Rain. The acidity or alkalinity of a solution is measured using the pH scale. pH levels range from 0 to 14, with 0 being extremely acidic. The scale is logarithmic, so a unit change in pH represents a tenfold change in acidity. A pH of 7 is neutral. Normal precipitation has a slightly acidic pH value of approximately 5.5. Acid rain forms due to the increased levels of sulfur dioxide and oxides of nitrogen that enter the atmosphere due to burning of high-sulfur content coal. These gases dissolve in the cloud drops, making the precipitation more acidic by a factor of ten, since one unit of change in pH means a ten-fold change in acidity. Industrial sources and petroleum-powered vehicles emit massive quantities of sulfur dioxide and nitrogen oxides into the atmosphere. The United States alone emits approximately 40 million tons per year. These chemicals undergo complex changes when in the atmosphere. Some get dissolved in raindrops, snow, or fog particles and produce weak solutions of sulfuric and nitric acids. As these acids fall to the ground they can accumulate in lakes and can affect ecosystems. For example, there has been a decline 15-14 in the health of coniferous forests in Appalachian Mountains from North Carolina to New England. In North America, acid rain is primarily in the Northeast and Canada, downwind of sources of sulfur dioxide and nitrogen oxides. Figure (15.13) represents the pH level of precipitation measured over the United States and Canada. Notice the low values of pH east of the Mississippi, with the lowest pH values (most acidic) occurring in northeastern United States and Canada. The situation is improving with the 1990 Clean Air Act amendments that mandate reductions in sulfur and nitrogen acid-forming compounds over the next 50 years. Acid rain is an example of how human activities that emit gases into the atmosphere can impact the environment. Output of greenhouse gases is another example of how human activities impact the environment. Greenhouse Gases and Clouds Greenhouse gases are important ingredients that determine the radiative energy balance of the planet and contribute greatly to determining Earth's average temperature. The primary greenhouse gases are water vapor, CO2, methane (CH4), and CFCs (chlorofluorohydrocarbons). Human activities are changing the concentration of these gases. The concentration of CO2 is increasing at a rate of approximately 0.4 percent per year. Since the beginning of the Industrial Revolution in the 18th century, concentrations have increased by 25%. Methane has doubled over the same time period. The current rate of increase in methane is about 1% per year and CFCs have increased at an even greater rate. 15-15 In the long term, Earth must emit energy to space at the same rate at which it absorbs solar energy. As discussed in Chapter 2, the atmosphere and surface absorb solar radiation and the rest is reflected back to space. Earth emits radiation to space in infrared wavelengths. Greenhouse gases prevent some of this radiation from escaping into space. As human activities increase the amount of greenhouse gases, the global energy balance of the Earth system appears to be perturbed. To maintain an energy balance, Earth's climate must adjust to get rid of the extra energy trapped by anthropogenic greenhouse gases. Global warming increases the amount of energy Earth radiates to space. Water vapor and clouds play a vital role in preserving the balance between incoming and outgoing energy. More water vapor molecules exist in warm air than in cold air. Water vapor is a strong greenhouse gas. Water vapor is transparent to solar radiation while being extremely effective at absorbing terrestrial radiation emitted from the earth's surface. Increased water vapor concentration leads to warmer atmospheres and a further increase in atmospheric water vapor. Human activities add little water vapor to the troposphere, at least directly. Water vapor increases are a result of internal controls of the climate system. In addition to warming, increased water vapor amounts may enhance cloud cover that can induce a global cooling. It is difficult to predict the effect of changes in cloudiness on climate. Different cloud types affect the climate in different ways. High thin cirrus can lead to a warming, similar to the greenhouse warming. Thick stratocumulus cause a cooling by reflecting large amounts of solar energy back to space. Satellite observations indicate that the average global cloud distribution causes a net cooling of the planet. However, we don't know which cloud types might predominate in a different climate scenario. 15-16 Aerosols can also affect climate by causing changes in cloud radiative properties. Aerosols serve as cloud condensation nuclei (CCN - Chapter 5). When more CCN are present, more droplets will form in the cloud, and they will be smaller. This makes the cloud more reflective, further reducing the solar energy reaching the surface. Observations of cloud droplets over the Atlantic Ocean downwind of Northeast North America, tend to be composed of drops that are smaller than similar clouds that are in a pristine environment. Evidence of this indirect aerosol effect is seen in satellite images of ship-tracks (Figure 15.15). Effluents from the ship engines rise upward into the cloud and serve as CCN. This increases the number of cloud droplets in the cloud, making it appear brighter. Smaller particles in high concentrations also make it less likely the cloud will yield precipitation. In addition to changing cloud properties, human activities may directly change cloud amount (Box 15.3) The effect of aerosols on the radiation budget through their impact on clouds is called an indirect aerosol effect on climate. The aerosols indirectly affect climate by changing cloud properties, such as cloud amount or particle size, that then changes the energy budget of the region. Ozone In addition to strongly absorbing radiation at ultraviolet wavelengths (Chapters 1 and 2), ozone also absorbs and emits electromagnetic radiation at wavelengths in the vicinity of 9.6 microns. So, Ozone is a greenhouse gas; however, its impact on our health is far more important than absorption of terrestrial radiation. The impact of ozone on health is determined by whether changes in ozone are occurring in the troposphere or stratosphere. 15-17 Ozone primarily occurs in the stratosphere though some ozone, approximately 10% of the total amount, exists in the troposphere. The maximum ozone concentration is between 20 and 25 kilometers (about 12 to 15 miles) above the surface. The layer of maximum ozone concentration in the stratosphere is referred to as the ozone layer. The altitude of the ozone layer varies with latitude. Stratospheric ozone is beneficial to life because it absorbs ultraviolet radiation coming from the sun that is biologically damaging. Absorption of UV energy heats the atmosphere and is responsible for the temperature inversion observed in the stratosphere. First we will re-visit stratospheric ozone hole and then discuss changes in tropospheric ozone concentrations. Stratospheric Ozone Hole As you learned in Chapter 1, ozone (O3) is produced by the combination of three oxygen atoms (O). The concentration of atmospheric ozone is small, approximately 3 molecules of ozone for every ten million air molecules. Through absorption of ultraviolet radiation (UV), ozone plays a fundamental role in the radiation budget and the dynamics of life on earth. Absorption of UV energy causes a heating that produces the increasing temperature with altitude, a characteristic feature of the stratosphere. Ozone absorption of UV also keeps this harmful radiation from reaching the surface. Reduction in amounts of ozone can lead to increase amounts of biologically damaging UV-B radiation (radiation with wavelengths of .2 to .32 microns) at the surface. Increased amounts of UV-B can lead to incidents of cataracts and skin cancers known as melanoma. For every 1 percent increase in UVB there is likely to be a 2 percent increase in the risk for contracting melanoma. 15-18 The total amount of ozone in an atmospheric column at a given location is measured in Dobson Units (DU), named after Dobson who developed methods of measuring atmospheric ozone from the ground. Dobson units represent how thick a layer of ozone from the surface to the top of the atmosphere would be if it all existed at 0C and the average surface pressure. 300 Dobson Units is 0.3 cm thick or approximately the thickness of a dime. Observations of the monthly average total column ozone amounts show a minimum amount over the Southern Hemisphere in spring. During winter, the amounts of ozone over the South Pole region remain fairly constant. A decline in ozone is seen in September and a minimum amount of ozone is observed in October. After October, ozone levels begin to increase. Why does the minimum occur in October? The winter atmosphere above Antarctica is very cold. These cold temperatures result from the high altitude of the Antarctic continent and the resulting energy losses due to emission to space of longwave radiation. Temperatures in the stratosphere can be less than -90C (-130F)! In addition, the cold temperatures result in a temperature gradient between the Southern Hemisphere polar and the midlatitudes. The temperature gradients result in pressure gradient that, in combination with the Coriolis force produces a belt of strong stratospheric winds that encircle the South Pole region. The strong westerly winds, referred to as the polar vortex, prevent the transport of warm equatorial air to the polar latitudes. This isolation between the south polar regions and midlatitudes and tropical regions helps keep the stratospheric air very cold. These cold temperatures cause water vapor and some nitrogen compounds to condense and form clouds. These polar stratospheric clouds, or PSCs, are composed of ice and frozen nitrogen particles and form 15-19 in air temperatures colder than approximately -80C or -112F. PSCs begin to form during June, and dissipate in October, the Antarctic spring. Chapter 1 mentioned that Cl is an important atom that contributes to the destruction of ozone. In the winter time chemical reactions on the surface of the particles composing PSC result in chemical reactions that bind the Cl into the PSC. During the spring, when sunlight again shines on the Antarctic stratosphere, chlorine atoms are freed and ozone is rapidly depleted. Destruction is so rapid over the South Pole region in the Southern Hemisphere springtime (e.g., October) that it has been termed a "hole in the ozone layer." Observations of ozone concentrations over Antarctica during October reveal a decreasing trend since 1975 (Figure 15.16). Observations of total ozone amounts in the month of October measured over Halley Bay, Antarctica (76 degrees south latitude) demonstrate this trend. There is a strong year-to-year variation in the development and size of the Antarctic ozone hole. This is demonstrated by plotting the October monthly average ozone amounts over the Antarctic during the periods 1980-1991 (Figure 15.17). Notice the reduced ozone amounts in 1987, 1989, 1990 and 1991. Why does the ozone hole appear more often over the South Pole than the North Pole? Stratospheric clouds composed of ice particles exist over Antarctica but not over the mid-latitudes or tropical regions. This is because the Arctic stratosphere does not get as cold as the air over Antarctica. As a result, while ozone depletion of 15-20% have been observed in certain regions of the Arctic stratosphere, the development of an ozone hole has not been observed. 15-20 Tropospheric ozone Ozone is a chemically active molecule and is considered a corrosive gas. High levels of ozone can damage plant and animal tissues when they come into contact with ozone due to chemical reactions. When atmospheric ozone is present near the surface it is a pollutant. High concentrations of ozone reduce crop production and are detrimental to human health. Thus, while decreased amounts of stratospheric ozone are dangerous to human health, decreased amounts of tropospheric ozone near the surface are beneficial! Ozone is a major component of photochemical smog, a type of air pollution that forms during sunny days when vehicular traffic is congested. In the presence of sunlight, oxides of nitrogen from engine exhaust and hydrocarbons react to form a noxious mixture of aerosols and gases. This mixture includes ozone, formaldehyde, and PAN (peroxyacetyl nitrates). Exposure to high concentrations of ozone irritates the eyes, nose and throat, and causes coughing, chest pain, and shortness of breath. Ozone also aggravates diseases such as asthma and bronchitis. Ocean-feedbacks There are two important reasons why the oceans are critical to understanding climate fluctuations. First, oceans occupy 70% of the Earth's surface and are the major source of atmospheric water vapor. Water vapor is intimately related to heat exchanges, and heat exchanges between the atmosphere and the large ocean surface determines atmospheric conditions. Second, the oceans have a large heat capacity. That is, compared to most other substances, it takes a relatively large amount of energy to change the temperature of water. This prevents extreme variation in global temperatures. 15-21 There are some obvious relationships between the atmosphere and ocean. Chapter 8 discussed these intimate relationships in terms of El Niño, La Niña, and hurricanes. Another relationship exists between water temperature and sea level. If the oceans warm, they expand causing a rise in sea level. Increases in atmospheric temperatures may also melt glaciers back into the oceans, increasing the sea level. If the West Antarctic ice sheet melted, sea level would rise by 5 m or more. There is evidence that the global sea level has risen by 20 cm (7.9 inches) during the last 100 years. Changes in sea level can change climate patterns by changing ocean circulation patterns. If oceanic temperature changes are occurring due to changes in atmospheric temperatures, it could take decades to observe these changes. If the oceans warm, it could modify atmospheric pressure distributions and wind patterns. In addition to serving as a heat reservoir, oceans moderate atmospheric temperatures by removing CO2 from the atmosphere. Table 16.2 lists the amount of CO2, N2 and O2 in the atmosphere and oceans. The amount of CO2 in the oceans is 500 times more than that in the atmosphere! We do not know how much more CO2 the oceans can absorb. If they can absorb more atmospheric CO2, they will modify the global warming. However, water can hold less gas at higher temperatures. If the oceans warm up, some of the currently dissolved CO2 would diffuse out to the atmosphere, increasing atmospheric concentrations. Predictions about climate change need to account for changes in temperatures of the Earth’s oceans. Ocean currents transfer heat from one place to another. Global-scale ocean circulation patterns transport heat poleward. As discussed in Chapter 8, surface currents in the Atlantic transport heat poleward. These currents cool as they move northward and 15-22 sink in the North Atlantic. They then flow southward at great depths to Antarctica Figure 15.18 demonstrates how North Atlantic Deep Waters are circulated through the ocean. This "conveyer belt" transports about 20 times more water than all of the world's rivers do in about 1000 years. The surface water in the North Atlantic near Greenland and Iceland is cooled through interactions with the atmosphere. The water sinks and flows south around Africa and northward in the Indian and Pacific Oceans. In the Indian and North Pacific Oceans the water mixes to the surface and enters the surface layer current, eventually bringing warm surface water to the Atlantic Ocean. . A global warming might affect the energy transport of the ocean by changing water temperatures in North Atlantic. There is convincing evidence that these flow patterns can change abruptly. Changes in ocean circulation are found in a marine record that appears at nearly the same time as swings in the Greenland air temperature. Evidence of past climates indicates that these changes could trigger a change in atmospheric temperature of more than 5C (9F) over a period of less than half a century! For example, the Younger Dryas cold period (Chapter 14) occurred at a time when the North Atlantic Deep Water circulation slowed. There is concern that a modest global warming can cause a rapid change in the ocean circulation pattern over the next century. Table 15.2 Gases in the ocean and atmosphere. Percentage by volume in Atmosphere Nitrogen 78.03 Percentage by volume in Ocean 47.50 Oxygen 20.99 36.00 Carbon Dioxide 0.03 15.10 15-23 Changes land surfaces As discussed in Chapters 2 and 3, the Earth's surface has a direct effect on regional weather and climate. Large-scale changes in land use, due to urbanization, deforestation, and farming, affect the surface of Earth, which can cause climate changes. These, in turn, affect the biosphere. The biosphere is comprised of all of Earth's living organisms. The biosphere plays an important role in the climate. It helps to regulate the carbon cycle, the hydrological cycle and the heat budget of the planet. Climate changes are linked to changing land-vegetation patterns. The influence of climate on vegetation is straightforward. Indeed, a climate classification system can be based on the types of plants of a region. Plants also influence the climate they live in. Examples of how changes in the surface, caused by a combination of human activity and natural variations in weather, impact on the condition of the ecosystem include the Dust Bowl, desertification, and urbanization. First we consider the Dust Bowl. Dust Bowl The climate of a region includes year to year variations in the weather. This is why we cannot attribute a single warm winter to global warming. We have to look at trends over many years. Sometimes abnormal weather persists for a few years or even a decade, after which the variations return to near normal. We can think of these shifts in weather as a climate fluctuation. The Dust Bowl of the United States is an example of a climate fluctuation. The Dust Bowl of the 1930's removed unprotected topsoil from productive farmland. It resulted from a combination of years of drought combined with a misuse of 15-24 land as grasslands that had been plowed for wheat in the 1920s were abandoned or returned to grazing. At its greatest extent the Dust Bowl region covered 25,000 sq. mi. (64,750 sq. km) of the southwestern Great Plains (Figure 15.19). Millions of hectares of farmland went to waste, and hundreds of thousands of people were forced to leave their homes. It was an ecological disaster, but was not a long-term change in the climate. Plotting the June, July, and August average temperature and rainfall of Topeka, Kansas can illustrate the climate fluctuations associated with the Dust Bowl for the 100year period between 1890 and 1990 (Figure 15.20). Notice the large variations in the observed year-to-year temperature and rainfall. In the mid 1930s there are a few years where there is a warm departure in summer temperature as well as a dry spell. There is no simple explanation of the cause of these hot and dry summers. While these are probably a natural fluctuation in the climate system, inappropriate land use could have enhanced the departures. Prior to World War I farmers plowed up the natural grasses to make way for farmland. This set the stage for the Dust Bowl years. A severe drought occurred in the 1930s. While the natural grasses had adapted to extended dry periods, the lack of precipitation denuded the surface of the agricultural plants. As the land dried up, the winds blew the topsoil away because of the lack of vegetation. The winds could sometimes generate dust storms that would darken the skies. The Dust Bowl lasted about a decade. Since the Dust Bowl, new cultivation methods designed for dryland ecosystems were developed which has reduced the impact of subsequent droughts. During the mid-1930's shelter belts where planted to stabilize the soil. A shelterbelt consists of plants, often a mixture of conifer and deciduous tress, in 15-25 rows perpendicular to the prevailing wind direction. As winds blow through the trees, friction increases and the wind is reduced downwind for a distance of about 25 times the height of the trees composing the belt (Figure 15.21). When properly planted, a shelterbelt keeps the wind from lifting the soil and transporting it downwind. Irrigation, replacing grasslands, contour farming, and other conservation measures are now widely used to mitigate the impact of droughts in this region. Desertification Desertification is the process of reducing the productive potential of arid or semiarid land Desertification refers to the spreading of a desert region due to a combination of climate change and human impacts on the land. due to a combination of climate change and human mismanagement. Practices that leave the land susceptible to desertification include: over grazing, deforestation without reforestation, and farming on land with unsuitable terrain or soil. The consequences of desertification include a magnification of the drought, a decline in the standard of living, and even famine. The semi-arid fringes of the Sahara Desert are vulnerable to desertification. The Sahara Desert is the largest land desert in the world. Just south of this desert, between 14N and 18 N is the Sahel. Below the Sahel are grasslands and then tropical forests. The Sahel, or Sub-Sahara, is a semi-arid region (climate type Bsh) with pronounced wet and dry seasons. The amount of rainfall is variable from year to year and from region to region. In winter, the Sahel is hot and dry. The Intertropical Convergence Zone (ITCZ) brings precipitation as summer approaches and the band of convective precipitation moves northward. The year-to-year variation in precipitation depends on the movement of the ITCZ and varies considerably. 15-26 In the early 1960's, rainfall was plenty and the nomadic people who live in this region of the world found ample grazing land for cattle and goats. Herds grew in number and so did the human population. In 1968 the ITCZ did not bring the needed rains as far north, marking the beginning of a severe drought that lasted into the 1980s The drought, in concert with overgrazing, turned large regions of pasture into a wasteland. The drought peaked in 1973 when rainfall totals where more than half the long-term average, and extended into the 1980s. The Sahara desert moved southward into the Sahel and a famine ensued that took the lives of more than 100,000 people and affected more than 2 million. Rainfall has returned to the region but not to the levels of the 1950s and 1960s. It is possible that this change in regional precipitation is a natural variation, Indeed, the southern boundary of the Sahara shifts position north and south by 100 km (60 miles). However, the drought may also be enhanced due to a bio-geophysical positive feedback mechanism The Sahel lies below the descending branch of the Hadley during its dry season. Sinking air warms and is thus an energy gain for the atmosphere. With a reduction in rainfall there is less vegetation. The reduced vegetation results in an increase in surface albedo, reducing the energy gains of the surface The energy gains of the atmosphere are also reduced as there is less transfer of heat from the surface to the atmosphere. To make up for this reduced energy gains from the surface, the atmosphere subsides. The sinking motion of the atmosphere compresses the air and warms and dries the air. This warming and drying associated with the sinking motion of the atmosphere further enhances desert conditions. Thus, the reduced precipitation of a semi-desert 15-27 region is a positive feedback mechanism. The drought reduces vegetation amount resulting in small energy gains resulting in subsidence that enhances the drought. Urban Heat Island It is a well-known fact, and has been for some time, that cities are generally warmer than the surrounding, rural areas. Cities are called urban heat islands. The reason the city is warmer than the country comes down to a difference between the energy gains and losses of each region. Urban heat islands are a good model with which to explore how changing the energy balance of a region can affect its temperature, its macroclimate. There are a number of factors that contribute to the relative warmth of cities, heat from engines, thermal properties of buildings, and Urban heat island refers to the increased temperatures of urban areas compared to a city’s rural surroundings. evaporation of water. The heat produced by heating and cooling city buildings and running planes, trains, buses and automobiles contribute to the warmer city temperatures. Heat generated by these objects eventually makes its way into the atmosphere, adding as much as one-third of the heat received from solar energy. The thermal properties of buildings and roads are also important in defining the urban heat island. Asphalt, brick, and concrete retain heat better than natural surfaces. Buildings, roads, and other structures add heat to the air throughout the night and thus reduce the nighttime cooling of the air, so that the maximum temperature difference between the city and surroundings occurs during the night. The canyon shape of the tall buildings and the narrow space between them magnifies the longwave energy gains. During the day solar energy is trapped by multiple reflections off the many closely spaced, tall buildings reducing heat losses by longwave radiation (Figure 15.22). 15-28 Pollution in the city's air also modifies the absorption of longwave and shortwave radiation of the atmosphere. Evaporation of water may also play a role in defining the magnitude of the urban heat island. During the day in rural areas, the solar energy absorbed near the ground evaporates water from the vegetation and soil. Thus, while there is a net solar energy gain, heating is lessened to some degree by evaporative cooling during evapotranspiration. In cities, where there is less vegetation, the buildings, streets, and sidewalks absorb the majority of solar energy input. The urban heat island is clearly evident in statistical tables of surface air temperatures (Figure 15.23). The warmer temperatures of urban areas are also apparent in cloud-free satellite images. Figure 15.24 is a satellite infrared image of radiative energy exiting the atmosphere. The image is similar to what you see on the television weather but with finer details. At this wavelength, the satellite instrument is measuring the amount of radiant energy emitted by the surface and the tops of clouds, which is proportional to the temperature of the emitting body. The warmer the body, the greater the amount of radiant energy emitted. White portions of the image represent cold objects (e.g., cloud tops) and dark regions are warm areas. A map is shown to help orient you to the geography of the region. Notice that on this day in April, the land is warmer (it appears darker in the image) than the Great Lakes and the Atlantic Ocean. Urban heat islands appear on the image as “dark blemishes.” Climate Modeling Climate results from a balance between interacting physical processes that occur in the oceans, atmosphere, land, or biosphere. Figure 15.25 is a simple illustration of the 15-29 complexity of our climate system. . A change in any of these processes illustrated in this figure could have an impact on the climate system. To assess how human activities might impact future climate we must understand how these physical processes interact to produce today's climate. Separating the human impact from any natural fluctuation is a major scientific challenge. To accomplish this requires careful monitoring of trends, such as those shown in Figure 15.1. Observations are important, but they will never be perfect. We are not able to measure everything, everywhere, all the time. To synthesize the measurements requires us to combine our observations with appropriate methods to forecast climate change. An important tool for forecasting climate changes is the Global Climate Model, or GCM. GCMs solve mathematical equations that express Global Climate Model (GCM) refers to a computer program that calculates global climate using mathematical equations derived from physical principles. physical laws, such as the conservation of energy (Chapter 2) and Newton's Laws of Motion (Chapter 6), and all the relationships discussed throughout this book. All these equations cannot be solved exactly, so computers use approximate solutions using finite differences. The roots of today's GCMs go back to the methods first developed by L. F. Richardson as discussed in Chapter 13. Different models make different approximations to finding these solutions. So, models tend to differ in their predictions. Accurate GCMs must include the behavior of the oceans and the biosphere as well as atmospheric processes. The interactions between the atmosphere, biosphere, oceans and land, as discussed throughout this book, are what make climate prediction so difficult. However, the ability of GCMs to account for current climate conditions as well as past climates lends confidence to predictions by 15-30 GCMs. As with today's weather models, climate models continue to improve and are essential in understanding changes in our atmosphere. Models are also valuable in identifying the fingerprints of global climate change. The impact of volcanic eruptions on changes in surface temperature was confirmed with observations and model predications of surface temperature after the eruption of Mt. Pinatubo. Until recently there have been limited observations of climate changes in the polar regions. Climate models have indicated that polar regions are very sensitive to a global climate warming. As a result, new efforts have been implemented to study the climate of the poles and document current changes. Models are also very valuable in understanding feedbacks and helping us to separate natural variations from human activities. A model can be used to determine how sensitive climate is to a given process. For example, if we want to understand how a change in cloud cover impacts climate, we can "force" a change in cloud amount in a climate model and analyze the model's response to this simulation. Or, we can fix the amount of cloud and change the vertical distribution of clouds. The climate model is the atmospheric scientists' laboratory to run controlled experiments! Such an experiment is shown in Figure 15.26?. The observed trend in global average surface temperature between 1860 and 1990 is shown. The dotted line shows the prediction of the global change using a GCM that allows for increases in greenhouse gases only. In the second experiment, the GCM allows for increases greenhouse gases and increases in sulfate aerosols. A comparison of these two runs with observations demonstrates the importance of sulfate aerosols in offsetting the greenhouse warming. 15-31 Climate models have been used to assess how increased amounts of CO2 impact on climate by allowing us to increase the amount of CO2 in the model atmosphere and have the GCM predict the atmosphere's response. A typical experiment used by climate modelers is to increase the amount of greenhouse gases in the atmosphere to those levels expected by the year 2050 and see how the model responds to these changes. Models predict a warming, though the degree of warming varies with the model used (Figure 15.26). This range can be thought as representing an uncertainty in our understanding of the atmosphere's response to changes. GCMs predict that the global mean temperature by the year 2100will be warmer than today by 1C (2F) to 4.5C (8F). It was through this type of modeling study that we learned the importance of water vapor as a greenhouse gas. GCMs are also critical in deciphering the impact of cloud-feedbacks on climate. GCMs consistently predict that the warming would be greater at the poles than the tropics, and that continents will warm more than oceans. The effects of this warming for humanity depend on the speed of the warming. Changes in temperature and precipitation may cause agricultural zones to move northward. Adapting to a rapid shift in agricultural zones could take decades, while an adaptation by natural ecosystems may take centuries. GCMs are certainly in need of improvement. For example, most GCMs are only now learning how to properly include the role of oceans. Models also do not accurately portray local conditions. It is likely that future climate change and fluctuations will result from a complex interaction of differing factors--GCMs will help us unravel the relationship between cause and effect. Finally, GCM simulations of climate change due to anthropogenic activities indicate what to look for in observations of the atmosphere. 15-32 While variations exist, many GCMs predict consistent qualitative changes (Figure 15.27) in the global distributions of temperature and the hydrological cycle. For example, while the troposphere is expected to warm, the stratosphere is predicted to cool. Temperature changes in the troposphere are expected to be greatest in the Northern Hemisphere winter. In addition, the land nighttime air temperatures are expected to rise faster than the daytime temperatures. Recent analysis of temperature observations supports these predictions. 15-33 Summary The forces and dynamics that produce Earth's climates are complex. Climates are related to latitudinal differences in energy gains and energy losses. If the energy gains or losses of a region are slightly modified, its climate can undergo a series of changes. Recent observations indicate that the Earth is warming. Human activities may play a role in this warming as it is accompanied by rapidly increasing concentration of atmospheric greenhouse gases. Greenhouse gases play a crucial role in determining the Earth's climate by affecting the energy budget of the planet. Burning of fossil fuels is increasing the amount of carbon dioxide in the atmosphere. Coal mining, intensive agriculture and leaky natural-gas lines yield higher methane concentrations. Methane is a greenhouse gas. CFCs, used in refrigerants and propellants in spray cans as well as many industrial processes, are greenhouse gases that also severely impact the protective ozone layer in the atmosphere.. Increases in any greenhouse gas can lead to a global warming. This warming, in the absences of other changes, increases evaporation that in turn increases the amount of water in the atmosphere. Since water vapor is also a greenhouse gas, the warming is enhanced. Clouds can lead to a cooling or a warming, depending on the type of cloud. This is because clouds have opposite effects on the solar and terrestrial radiation budgets. Clouds tend to reduce the amount of solar radiation absorbed by increasing the albedo of the planet. In the longwave radiation, clouds increase the energy gains of the planet by reducing the amount of energy emitted to space. It is difficult to determine what will be the final outcome of cloud feedbacks on climate changes, since it depends on changes on 15-34 the amount of cloud, the type of cloud, as well as the size of the cloud droplets that the cloud is composed of. Natural temperature fluctuations occur on several time-scales and space-scales. Changes in the Earth's orbit around the sun are responsible for some climate fluctuations. The Milankovitch cycles combine to produce variation in the solar radiation received by Earth that correspond to the major ice ages discussed in Chapter 14. Volcanic eruptions can also cause climate changes. The recent eruption of Mt. Pinatubo resulted in a global cooling of 0.5C the year after its eruption. Natural variations complicate our ability to separate natural climate fluctuations from those caused by human activities. Unambiguous detection of climate change is therefore a slow process that must be done carefully and involve detailed comparisons of observations with climate model predictions. Climate variations are governed by changes in the atmosphere, but also by changes in the ocean, cryosphere, and the biosphere. The interaction between climate and a change in the land are complex. However, these interactions are sometimes measurable. For example, the expansion of a city modifies the energy and water budgets of the previously rural region. These changes have resulted in cities being warmer than the surrounding regions. As another example, severe overgrazing by cattle and sheep denude the land of vegetation. The loss of vegetation can greatly affect evapotranspiration and the heat budget of the surface, giving way to a permanent desert. In addition, removal of vegetation exposes the topsoil to erosion, as in the Dust Bowl of the U.S. Great Plains in the 1930s. 15-35 Predicting future climate trends over the next hundred years is a difficult task. Global Climate Models help us to better understand and predict climate fluctuations and changes by incorporating mathematical models that represent the physics, chemistry, and biology of the Earth. There are different models with varying complexity. Climate simulations using these models indicate that the Earth is warming, and will continue to warm over the next 50 years. Based on the results of different GCMs, the amount of this warming is uncertain. Predictions continue to be refined as we improve our understanding of the atmosphere and its relationship to the oceans and the biosphere. 15-36 Terminology You should understand all of the following terms. Use the glossary and this Chapter to improve your understanding of these terms. Acid rain Carbon Monoxide Desertification Dust Bowl Eccentricity Global Circulation Model Milankovitch cycles Negative Feedback Nitrogen Dioxide Obliquity Positive Feedback Precession Shelterbelt Sulfur Dioxide Urban heat island 15-2 Review Questions 1. Why are the average temperatures of cities often greater than the surrounding rural region? 2. Are there other differences in weather between the city and its surroundings? 3. How do the oceans influence climate? 4. Do you think a Dust Bowl can occur again? 5. What is a Global Climate Model? 6. What is an Urban Heat Island? 7. If you live in a large city, compare the observations of temperature in the city with those of the surrounding regions for a month. Is there any difference? 8. What is the most important gas that contributes to the greenhouse warming? 9. What is the difference between a positive feedback and a negative feedback? 10. What is desertification? 11. Explain why increasing the amount of stratus clouds over the oceans would result in a net radiative energy loss. 12. Explain why increasing the amount of cirrus clouds over the hot deserts would result in a net radiative energy gain. 13. What are the Milankovitch cycles and how do they cause variations in climate. 14. Provide two examples of a positive and negative feedback. 15. Why do thin cirrus tend to cause a warming while stratus clouds cause a cooling? 15-2 15-3 16. Describe how a slight increase in the horizontal extent of glaciers could cause the glaciers to grow? 17. Why are climate models useful for understanding climate change? 18. Why do some volcanoes cause a cooling of the earth while others have no impact? 19. Why would increasing levels of ozone in the troposphere be of concern to health officials? 20. What are advantages and disadvantages of using global climate models to predict future climate changes? Web Activities Simple climate models The Earth radiation budget experiment Forming Contrails Practice multiple choice exam Practice true/false exam 15-3 15-4 Box 15.1 Variations in Solar Output Recent measurements of the energy output of the sun indicate that the output varies slightly. The sun's energy output is correlated to sunspot activity. Sunspots are magnetic storms on the sun that appear as dark spots on the sun's surface (include figure of sunspot). Since sunspots are magnetic storms, the sun's magnetic field also varies with sunspot activity. Like magnets, sunspots have a polarity. The polarity of the sunspots reverses every 11 years, so the sun's magnetic cycle is 22 years. The number and size of the spots reaches a maximum every 11 years. Sunspot activity peaked in 1980 and 1991 and is expected to peak again in 2002. Recently, a minimum number of sunspots occurred in 1975, 1986 and 1997. Large numbers of sunspots reduces the solar output by only 0.1%. This change in total solar energy output is not very large, particularly in terms of modifying the weather . There is however, anecdotal and empirical evidence that links the sunspot cycle with Earth's climate. For example, a period of reduced sunspot activity was observed during 1645 to 1710. During this time there were few, if any sunspots. This is period is called the Maunder Minimum and it occurred at the same time as the Little Ice Age. There are also fluctuations in weather patterns of the Northern Hemisphere that coincide to an approximate 22-year cycle. For example, there is a periodic 20-year drought in the Great Plains of the United States. So, any correlation between sunspot number and climate fluctuations is likely to include some feedback process. As of yet, scientific explanations of the 22-year correlation are have not been fully proven. The peak in sunspot activity affects the solar wind that bombards our upper atmosphere with high-energy particles. At a peak of sunspot cycle the upper atmosphere 15-4 15-5 can reach temperature of1225C (2240F), whereas during a sunspot minimum the temperature is only 225C (440F). It is not fully understood how these upper atmospheric changes can affect the troposphere. 15-5 15-6 Box 15.2 Atmospheric Pollutants Air pollution is defined as airborne solids, liquids, or gases that, when in high concentrations, threaten the lives of people and animals, harm plants, or threaten ecosystems.. Pollutants can arise from human activities (anthropogenic sources) or from natural sources (such as, dust storms and volcanic eruptions). Human activities can both enhance and degrade our quality of life. Air pollution consists of the undesirable gases and particulates emitted into the atmosphere by humans. Humans create two basic types of air pollution, primary pollutants and secondary pollutants. Primary air pollutants are those directly emitted from a source. Examples are carbon monoxide from automobile engines or smoke from burning rainforests. Secondary pollutants form as a result of chemicals produced by humans that subsequently react in the atmosphere with harmful results. Examples are tropospheric ozone and acid rain. Carbon monoxide (CO), a colorless and odorless gas, is an example of a primary pollutant. CO is very toxic as it disrupts how red blood cells absorb oxygen. As a result, inhalation of CO reduces the body's ability to provide oxygen to the body. CO gas is also a byproduct of incomplete combustion. Automobiles are a source of CO. In cities, the CO levels can approach unsafe levels in confined areas, such as garages and tunnels. CO is also very dangerous in the home where it can be produced by heating devices that are not operating properly. Secondary pollutants, can develop from harmless chemicals that are emitted directly into the atmosphere, but become a noxious gas or particulate after chemically combing with other atmospheric constituents. 15-6 15-7 Human activities produce oxides of sulfur, in particular sulfur dioxide (SO2) and sulfur trioxide (SO3). These sulfur oxide compounds are released into the atmosphere primarily through the burning of fossil fuels that contain sulfur. Levels can become large when the activities concentrate the compounds over a small region that allows the pollutant to reach high levels, such as urban and industrial areas. SO2 is a highly corrosive gas that irritates the human respiratory system. SO3 is an important secondary pollutant as it readily combines with water vapor to form droplets of sulfuric acid (H2SO4). This combination can result in and acid fog, or acid precipitation (i.e. acid rain or acid snow). Acid fog is particularly dangerous to people, as it can be easily inhaled. Acid precipitation affects the environment in falls on, depositing the sulfuric acid into the lakes, streams, ground water, and the soil. Over time, the surface water system becomes acidic and can become threatening to vegetation and animal life. Acidification can be high enough to render lakes barren of fish. This is a particular problem in the eastern United States, and eastern Canada. The problem is even more severe in Norway and Sweden where an estimated 6500 lakes are essentially lifeless. Much of the sulfur that comprises the acidic rain is produced upwind of the affected region. Oxides of nitrogen, in particular nitric oxide (NO) and nitrogen dioxide (NO2) are two important air pollutants. NO is a byproduct of high temperature combustion, such as in automobile engines and electric power generation. Indeed, atmospheric concentrations of NO2 in urban areas are well correlated with the density of vehicular traffic. NO is a very reactive gas and quickly forms NO2. NO2 is also a toxic gas that is also emitted by 15-7 15-8 automobile engines. High concentrations of NO2 give polluted air its reddish-brown color. In high concentrations, oxides of nitrogen cause serious pulmonary problems. Hyrdrocarbons are compounds made of hydrogen and carbon atoms. Example hydrocarbons are methane, butane and propane, which can occur as either a gas or particulate. Hydrocarbons are also called volatile organic compounds (VOC). Hyrdrocarbons do not appear hazardous in themselves, but , during the day, they can combine with nitrogen oxides and oxygen to produce photochemical smog. Air pollution is not a modern age problem. Cave dwellers undoubtedly had to deal with local air quality problems such as smoke from fires. The Hopi Indians had to deal with SO2 emitted from burning coal to make pottery. England has long had a problem with coal burning. Infant mortality in the late 18th century was alarming due to the burning of high sulfur-content coal. Air quality was so poor, that it has been estimated that half the children died before their 2nd birthday 15-8 15-9 Box 15.3 Contrails The white condensation trails left behind jet aircraft are called contrails (CONdensation TRAILS). Contrails form when hot humid air from jet exhaust mixes with environmental air of low vapor pressure and low temperature. The mixing is a result of turbulence generated by the engine exhaust. Cloud formation by a mixing process is similar to the cloud you see when you exhale in cold air and "see your breath". The figure below represents how saturation vapor pressure varies as a function of temperature. The blue line is the saturation vapor pressure for ice as a function of temperature (in degrees Kelvin). Air parcels in the region labeled saturated will form a cloud. Imagine two parcels of air, A and B as located on the diagram. Both parcels are unsaturated. If B represents the engine exhaust, then as it mixes with the environment (parcel A) its temperature and corresponding vapor pressure will follow the dotted line. Where this dotted line intersects the blue line is were the parcel becomes saturated and a cloud forms. If you are attentive to contrail formation and duration, you will notice that they can rapidly dissipate or spread horizontally into an extensive thin cirrus layer. How long a contrail remains intact, depends on the humidity structure and winds of the upper troposphere. If the atmosphere is near saturation, the contrail may exist for several hours. On the other hand, if the atmosphere is dry then as the contrail mixes with the environment it dissipates. Contrails are a concern in climate studies , because increased jet aircraft traffic may result in an increase in cloud cover. It has been estimated that in certain heavy air-traffic corridors, cloud cover has increased by as much as 20%. An increase in cloud amount changes the region's radiation balance. For example, solar 15-9 15-10 energy reaching the surface may be reduced, resulting in surface cooling. They also reduce the terrestrial energy losses of the planet, resulting in a warming. Jet exhaust also plays a role in modifying the chemistry of the upper troposphere and lower stratosphere. 15-10