Properties of Carbanions/organometallic compounds: M-R
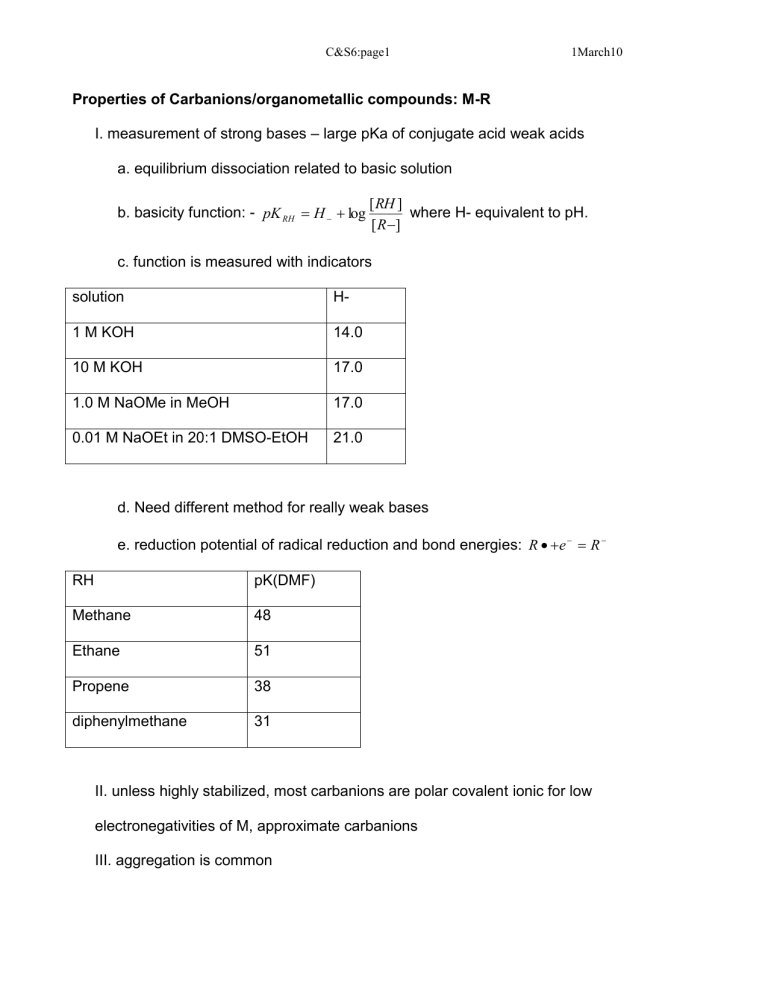
C&S6:page1 1March10
Properties of Carbanions/organometallic compounds: M-R
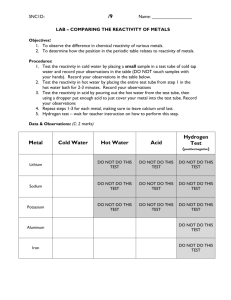
I. measurement of strong bases – large pKa of conjugate acid weak acids a. equilibrium dissociation related to basic solution b. basicity function: - pK
RH
H
log
[ RH ]
[ R
]
where H- equivalent to pH. c. function is measured with indicators solution H-
1 M KOH
10 M KOH
14.0
17.0
1.0 M NaOMe in MeOH 17.0
0.01 M NaOEt in 20:1 DMSO-EtOH 21.0 d. Need different method for really weak bases e. reduction potential of radical reduction and bond energies: R
e
R
RH
Methane pK(DMF)
48
Ethane
Propene
51
38 diphenylmethane 31
II. unless highly stabilized, most carbanions are polar covalent ionic for low electronegativities of M, approximate carbanions
III. aggregation is common
C&S6:page2 1March10 a. lithium reagent usually form clusters b. 2RMgX
R
2
Mg + MgX
2
R
2
Mg
MgX
2 solvent and R affects equilibrium, halide used affects dimer and cluster formation
IV. metals coordinate lone pairs of solvent
Generation of carbanions (see March Chapter 12)
I. metallodehalogenation: RX M RM X
RX
M
RX
M
RX R X
R
M
RM
'
' a. acid-base reaction b. R'M is often butyl lithium, LDA (lithium diisopropyl amide), could be KOH for stronger acids c. RH must be stronger acid but kinetics may not always be favorable d. PhH
BuLi
PhLi
BuH why is this favorable?
III. transmetallation with zero valent metal: RM M '
RM M
forward reaction favored when M more electronegative than M'
R Cd
2 Na
2
'
'
forward reaction favored when M' is more electronegative than M
2 RMgBr
HgBr
2
R
2
Hg
2 MgBr
2
V. transmetallation with other organometallic compound
R ' M
RM ' R ' M '
RM
C&S6:page3 1March10 general rule: strongest acid pairs with strongest base
(most electronegative acid pairs with most electropositive base)
'
' rare because of competition from Wurtz reaction, elimination
Reactivity/reactions of carbanions
I. reactivity of carbanion usually estimated from pKa of conjugate acid a. also use equilibrium RLi + R'I
RI + R'Li vinyl < phenyl < cyclopropyl < ethyl < n-propyl < isobutyl < neopentyl < cylcopentyl b. or R
2
Mg + R'
2
Hg
R
2
Hg + R'
2
Mg phenyl < vinyl < cyclopropyl < methyl < ethyl < isopropyl c measure of relative affinity for Li and I, Mg and Hg
II. gas-phase measurements give different results
III. stabilization by delocalization, ewg, s character, dipole
-stabilization NO
2
> COR > COOR > SO
2
R > CN
CONH
2
> X > H > R
O IV. carbanion reactivity depends on metal counter ion
Rates: Li + < Na + < K +
V. unconjugated carbanions are probably sp 3
M
+ EtI k
(a) can be formed at bridgeheads (unlike carbocations)
(b) invert rapidly
VI. vinyl anions retain stereochemistry
Reactions and Mechanisms
H
React with electrophiles: acid, S
N
2, displacement
Ph of leaving group, polar multiple bonds
Br Li
Ph
H
Ph Ph
CO
2
H
+
H
Ph
H
Ph
CO
2
-
Ph
H
Ph
C&S6:page4 1March10
I. D
E
A
E
(S
E
2) front side and backside attack
R
Hg
Br
HX
RH + XHgBr
II. cyclo-D
E
A
E
D n
A n
(S
E i)
R
M
M'
X RM' + XM most bimolecular substitutions occur with retention of configuration requiring front side attack and it is difficult to distinguish mechanisms
III. D
E
+ A
E
(S
E
1)
RM
R
M
RE
A. racemization, inversion or retention can occur depending on solvent and carbon anion formed
IV. Structure/Reactivity
A. S
E
2 backside attack slows with
branching
B. mechanism is usually S
E
2 or S
E i for electronegative metal (Hg, Cd, Zn, Si), and S
E
1 for electropositive groups (K, Na, Ca)
C. kinetic and thermodynamic control
O
O O O
LDA, -78 o
C
KH. 20 o
C
100%
42%
0%
46%
0%
12%
O
O
C&S6:page5
O
1March10
LDA, -78 o
C
KH. 20 o
C
99%
1%
74%
26%
Selected reactions
I. NH
2
can exchange alkane CH by S
E
1
II. base catalyzed enolization of carbonyls
HC
3
O
H
C
H
H
OH
-
HC
3
O
-
H
C
H
base catalyzed halogenation carbonyl
-CH
IV. oxidation of organometallics with X
2
and RXXR
RMgX
xsO
2
ROOMgX electron transfer!
ROOH , RMgX ROOMgX
2 ROMgX
V. R B
2 RNH
2
( ) (xs ammonia and hypochlorite)