Temperature, Heat and Internal Energy
advertisement

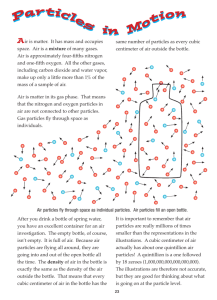
Temperature, Heat and Internal Energy (A) Temperature and thermometers Temperature is a measure of the hotness of a body. (1) Temperature scale and thermometer Temperature is expressed using a temperature scale. A temperature scale is obtained by choosing two fixed points, and dividing the range between them into a number of equal divisions called degrees. The most commonly used temperature scale is the Celsius temperature scale. Thermometer is a device for measuring temperature. Each type of thermometer uses a physical property that changes with temperature. For example, in a liquid-in-glass thermometer, the liquid expands and rises up in the narrow tube when temperature rises. (2) Temperature and Particle Motion According to the kinetic theory, all matter is made up of very tiny particles, which are constantly in motion. When they are close together, they attract/repel each other strongly. When they are far apart, they hardly attract/repel each other at all. Kinetic theory can be used to explain the general properties of solids, liquids and gases. Solids Liquids Gases General property Arrangement of particle Fixed volume and Particles are close shape together are arranged in fixed positions Fixed volume but Particles are also close no fixed shape together, but they are not in fixed positions. Movement of particles Each particle only vibrates to and fro about a fixed position. Each particle can move from one place to another. No fixed volume Particles are very far Each particle can move and shape apart. There is almost no from one place to attractive force between another. them. The average kinetic energy of particles in an object increases with temperature of the object. Two bodies have the same temperature if particles in each body have the same average kinetic energy. Temperature, Heat and Internal Energy 1 (B) Heat and internal energy When two bodies of different temperatures touch each other, energy is transferred from the hot body to the cold body until they reach the same temperature. (The bodies are then said to be in thermal equilibrium.) Heat is the energy transferred from one body to another as a result of a temperature difference. Unit of heat: joule (J) Internal energy is the energy stored in a body. It increases when the temperature of the body rises, or when the body changes from solid to liquid or from liquid to gas. Internal energy is the sum of kinetic and potential energy of all particles in the body. Unit of internal energy: joule (J) Power means the rate of energy transfer, i.e. Power Energy Time Unit of power: watt (W) or joule per second (J s-1) (C) Heat capacity and specific heat capacity (1) Heat capacity Heat capacity (C) is the energy transferred to raise the temperature of the object through 1 °C. Heat capacity = mass × specific heat capacity or C = mc Unit of heat capacity: J °C–1 (2) Specific heat capacity Specific heat capacity (c) is the energy transferred to raise the temperature of 1 kg of the substance through 1 °C. Energy transferred = mass × specific heat capacity × change in temperature or E = mcT Unit of specific heat capacity: J kg–1 °C–1 Temperature, Heat and Internal Energy 2 (3) Measuring specific heat capacity Measuring specific heat capacity of Measuring specific heat capacity of water aluminium Experimental set-up c Calculation E mT c E mT Do not switch on the heater Add a few drops of oil to the holes in unless its heating part is totally the aluminium block. immersed in water. Precaution Keep the heating part of the Place the aluminium block on a heater totally immersed in water polystyrene tile. throughout the experiment. Do not take the final temperature as soon as the power supply is Do not switch on the heater unless its heating part is in contact with the switched off. Stir the water and aluminium block. record the highest reading. Possible sources error of Some energy is lost to the Some energy surroundings. surroundings. is lost to the Some energy transferred is used to heat up the polystyrene cup, the stirrer and the thermometer. (4) Importance of high specific heat capacity of water Water is a good choice for use as a coolant. Coastal areas have cooler summers and milder winters than inland areas of the same latitude. The body temperature only changes slowly when the temperature of the surroundings changes. Temperature, Heat and Internal Energy 3