Deposition and characterisation of Ultrananocrystalline Diamond Films
advertisement

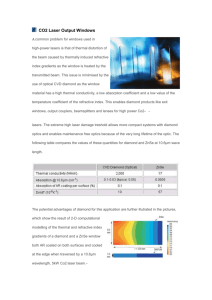
Molecular beam mass spectrometry of microwave activated plasmas used in diamond chemical vapour deposition. 1. Track Record Work at the University of Bristol in the area of diamond and other carbon-based thin films began in the early 1990s, and has since expanded rapidly. Our diamond and hard carbon film related work now encompasses both stand alone and collaborative projects involving research groups across the University. Chemistry, being the main centre for film growth, is a lynch-pin in many of these activities, and we have a number of deposition reactors capable of making a wide variety of carbon-based films ranging in properties from graphitic to polycrystalline diamond. Reviews of this work, placed in the context of work performed elsewhere, can be found in [1] and [2]. One major activity in the School of Chemistry involves studies of the fundamental physical and chemical processes underlying the chemical vapour deposition (CVD) of both diamond and diamond like carbon (DLC) films. To this end, we have developed a number of experiments devoted to investigating the gas phase chemistry and physics prevailing in successful diamond CVD. For example, we have measured the mole fractions of the more abundant gas phase species in the vicinity of the substrate surface during hot filament (HF) or microwave plasma enhanced (MW-PE) CVD of diamond using a first-generation, differentiallypumped, molecular beam mass spectrometer (MBMS) system, designed and assembled in 1994. We first used this system to probe the major species (CH4, C2H2, etc) in HF activated hydrocarbon/H2 mixtures, and the way in which their relative concentrations varied with process conditions, and then progressed to investigate the effects of trace additions of, e.g., halogens, nitrogen and PH3 on the gas phase chemistry and composition, and on the deposited film [3]. The same diagnostic technique was subsequently employed to study aspects of diamond film growth by MW-PECVD and to investigate the effect of varying the hydrocarbon, and of small additions of N-containing gases (which might act as potential N-dopants for diamond) [4]. This apparatus was also used to study low temperature diamond deposition using CO2/CH4 plasmas [5], and the effects of trace additions of H2S to this gas mixture [6]. This apparatus has been both productive and a source of considerable insight, but its performance is limited, and has deteriorated with time. Redesigning and greatly enhancing an apparatus of this type is a major objective of this proposal. A second experimental strand has involved the development and demonstration of optical and laser spectroscopy methods for in situ probing of transient species present during diamond CVD. Our first efforts in this area involved the application of resonance enhanced multiphoton ionisation (REMPI) methods for spatial profiling H atoms and CH3 radicals in a HFCVD reactor, as a function of process conditions [7]. This work provided the first consistent interpretation of the gas phase chemistry underpinning diamond growth from C2H2/H2 mixtures, and served to initiate our on-going (Royal Society and INTAS/NATO supported) collaboration with Dr Yuri Mankelevich and colleagues in the Nuclear Physics Institute at Moscow State University [8]. We subsequently received a DC arc jet reactor on long term loan from De Beers Industrial Diamonds (now Element Six Ltd) and began a series of progressively more sophisticated diagnostic and modelling studies of this rather unique environment for diamond CVD, at high growth rates (>100 m h-1). Initial studies of this plasma environment involved optical emission and absorption studies [9], but subsequent grants with colleague Prof Orr-Ewing (GR/M67506, GR/R71726), plus support from Element Six Ltd, have enabled the development and application of cavity ring down spectroscopy (CRDS) methods [10-12] for measuring spatially-resolved, absolute column densities of important radical species like C2, CH, C3, etc.) within this environment. This has served to trigger a major modelling effort; the level of agreement between experiment and model prediction [13] has now reached a stage where Element Six Ltd are keen to see such studies transferred to (industrially more relevant) MW-PECVD environments – see below. The DC arc jet was also a key component of a project funded as part of the Carbon Based Electronics (CBE) initiative (EPSRC GR/R97078) to investigate the deposition of nanocrystalline diamond (NCD). This material is currently attracting much interest, both because of its smoothness and its electronic properties. The CBE collaboration has enabled preliminary diagnosis of the plasma used for NCD deposition at high growth rates. This is such an exciting and topical area of materials research, however, that we see a great opportunity to extend these studies to MW-PECVD environments and MBMS analysis. This forms another aspect of the current proposal. Late last year, the applicants were awarded funding from the Royal Society/Wolfson Nanoscience Laboratory Refurbishment scheme. This funding, boosted by further expenditure from SRIF2, has enabled all 2 diamond related science in Chemistry at Bristol to expand into newly refurbished, clean, purpose-designed and properly-ventilated laboratory space, with contiguous desks and office space for 10 researchers. Previously, the applicants had also been successful in acquiring a state-of-the-art laser Raman system incorporating 3 laser wavelengths spanning the ultraviolet, green and infrared spectral regions under the JREI scheme (GR/R06069, with additional support from Renishaw plc and Element Six Ltd). This spectrometer is proving to be a remarkably useful diagnostic tool, enabling estimation of sp3:sp2 ratios and bonding characteristics in a diverse range of carbon films. As mentioned previously, the insights now flowing from the laser diagnosis/modelling studies of the arc jet environment has encouraged Element Six Ltd to design, build and donate a complete, state-of-the-art MW-PECVD reactor equipped with entry and exit ports for laser beams, thereby allowing us the opportunity to investigate high quality diamond growth under conditions and at growth rates of industrial relevance and applicability. As well as stand-alone projects, the group in Chemistry has a number of long standing interactions with colleagues in Physics and in the Interface Analysis Centre (IAC) at Bristol [14]. One project that is currently attracting much interest (and £1M funding from Advance Nanotech) involves collaboration with Prof Cherns and Dr Neil Fox and is aimed at developing and demonstrating doped nanodiamond coatings for low power, high brightness Field Emission Display (FED) applications. The IAC provides access to a number of specialised diagnostics ideal for analysing the various types of carbon films – including X-ray and ultraviolet photoelectron spectroscopy (XPS & UPS, respectively), secondary ion mass spectroscopy (SIMS), scanning Auger microscopy (SAM) and electron probe microanalysis (EPMA). The two applicants bring complementary skills and expertise in thin film deposition, materials analysis and gas phase diagnostics to the proposed project. The P.I. (PWM) was promoted to a Senior Lectureship in the School of Chemistry in 2002. He held a Ramsay Memorial Fellowship (1992-4) and a Royal Society University Research Fellowship (1994-9) prior to appointment to a Lectureship. Other relevant previous experiences include a PhD involving both experimental studies and modelling of reactive ion etching plasmas, and three years employment as a research scientist at GEC Hirst Research Centre. He has published over 80 papers/articles in refereed Journals, 60 of which are on the subject of diamond/DLC films. The co-applicant (MNRA) was promoted to a Chair in Physical Chemistry in Bristol in 1992, and is currently Head of the Physical and Theoretical Chemistry Section of the School of Chemistry. He is a past winner of the Marlow (1987), Corday-Morgan (1989), Tilden (1996) and the industrially sponsored award for Spectroscopy (2000) medals of the Royal Society of Chemistry, and was an EPSRC Senior Research Fellow during the period 1997-2002. His group has long-standing interests in many aspects of gas phase and gassurface chemistry, described in some 270 peer reviewed publications. He, together with Bristol colleagues OrrEwing and Western, and Oxford colleagues Hancock and Softley, were selected as one of the groups to pilot the EPSRC portfolio partnership scheme (GR/S71750). 3 2. Proposed Research Objectives: To build a second-generation molecular beam mass spectrometer for in situ, throughsubstrate sampling of a wide range of process gas mixtures and conditions used for diamond film growth in a microwave plasma enhanced CVD reactor. To use such measurements to tension and refine our current gas phase chemistry and reactor models so as to provide a more robust understanding of the gas phase and gassurface chemistry underpinning diamond CVD, and to enable prediction of future process optimisations. A. Introduction The last few years have seen numerous publications pertaining to the growth and characterisation of thin films of CVD diamond [1,15,16]. This is because diamond films have many attractive properties: they are hard, wear resistant, offer low friction, have high thermal conductivity, are electrically resistant and optically transparent over an unusually wide frequency range (mid-IR up to approaching the onset for the vacuum UV region). Additionally, their effective surface work function (electron affinity) is close to zero - or even negative [17]. The voltage required to cause electron emission from carbon surfaces in vacuum is thus very low, suggesting possible use in cold cathode emission devices, e.g. ultra-fast switches or FEDs. Consequently, diamond and DLC films (in all their many variants) are increasingly finding application in electronics [18], in optics and in engineering, with multi-million pound markets predicted. However, the race for applications has tended to hinder development of fundamental understanding of, for example, the chemical and physical processes occurring within the gas phase close to the substrate surface, and at the gassurface interface during CVD. Aspects of diamond CVD thus remain largely empirical. The diamond group at Bristol has a history of developing and demonstrating diagnostic methods for probing details of the gas phase chemistry prevailing in a diamond CVD reactor. Many of these involve laser spectroscopy, including, for example: resonance enhanced multiphoton ionization (REMPI) detection of H and CH 3 radicals in HF activated CH4/H2 and C2H2/H2 gas mixtures [2,8]. Currently, we are using this method to profile B atom number densities in HF activated B2H6/H2 and B2H6/CH4/H2 gas mixtures [19]. (B-doped diamond is a p-type semiconductor, and is finding ever increasing use in intrinsic devices, electrochemical sensors, etc. [20,21]) pulsed cavity ring down spectroscopy (CRDS) of C2(X and a states), and CH radicals, and electronically excited H(n=2) atoms in a DC arc jet reactor operating with CH4/Ar/H2 gas mixtures [11-13]. Our most recent achievements with this diagnostic include similar absolute column density measurements of C 2(a) radicals and H(n=2) atoms in the new microwave reactor provided by Element Six Ltd, and the first demonstrations of our ability to translate the optical cavity and thus determine vertical profiles (of C 2(a) radicals in the arc jet plume). direct line-of-sight diode laser absorption spectroscopy (DLAS) measurements in the DC arc jet reactor, e.g. of H(n=2) atoms in H2/Ar plasmas and of H(n=2) atoms and of C2H2 molecules in CH4/H2/Ar gas mixtures. first demonstrations of the application of a quantum cascade (QC) laser operating at ~1275 cm-1 for direct lineof-sight monitoring of CH4 and C2H2 in the new microwave reactor (with Prof Duxbury, Univ. Strathclyde). Each program of experimental work is complemented by modelling of the relevant gas phase chemistry in association with Dr Yuri Mankelevich of Moscow State University. In the case of the hot filament reactors, the DC arc jet reactor and, most recently, the 1 kW microwave reactor, the level of agreement between experiment and modelling is becoming extraordinarily good. However, a more detailed inspection of the list of species amenable to laser probing reveals a serious limitation. The probed species are generally atoms or diatomics. We, and others, have reported REMPI detection of CH 3 radicals in HF activated CH4/H2 gas mixtures [8], but no-one has yet reported successful detection of such a simple species as C2H, which we now predict to be one of the, if not the, most abundant C containing radical species incident on the growing diamond surface in both the DC arc jet and MW-PECVD reactors. This is principally due to problems of spectral congestion. The density of rovibronic fine structure in both the infrared and the electronic spectra of such species at gas temperatures ~3000 K precludes unique species identification and/or characterisation. We recognised the desirability of an alternative, universal, gas phase diagnostic as long ago as 1994, when we reported our first attempts at using MBMS methods to sample gas from an HF-activated CH4/H2 gas mixture [22]. The experimental challenge is to try and probe the gas while introducing the minimum possible perturbation to either the sampled portion itself or the volume from which the sample is extracted. In the apparatus we designed and built, we chose to sample the gas mixture in a region away from the substrate – to simplify interpretation. We sampled through a skimmer cone, into a differentially pumped volume – thereby creating some form of molecular beam (and thus reducing the frequency of unwanted collisions) – and then through a second orifice into the source region of a separately-pumped quadrupole mass spectrometer (QMS) equipped with a variable energy electron impact ioniser. The concept had much to commend it, and we were able to carry out a number of significant studies of HF activated hydrocarbon/H2 gas mixtures, and the effects of adding trace amounts of, for example, N (as N 2 or NH3), S (as H2S) and Cl, and to explore microwave activated CH4/CO2 gas mixtures. Stable species like HCN, CH3SH and HCl were readily detectable in these various gas mixtures, but the all important radical species generally defeated us. This unique apparatus had a number of limitations, which became most obvious when we began trying to probe commercially more important MW-PECVD environments. The skimmer cone was obtrusive; gas-surface chemistry occurred on it and it visibly perturbed the plasma. The pressure reduction stage was under-pumped, and the sampled gas 4 therefore suffered many collisions en route to the mass spectrometer. As a result, the process gas mixture was not ‘frozen’ during transit from the reactor to the mass spectrometer, and many radical species reacted away before detection. Finally, of course, we were sampling a region of gas that was not necessarily representative of the composition immediately adjacent to the growing diamond surface. Recent results from a group in Hungary [23] using ‘through-the-substrate’ sampling (i.e. where the gas had the opportunity for further processing via gas-surface encounters) suggest species mole fractions differ significantly in detail from those obtained using our previous geometry. At this stage it is not totally clear to what extent these are absolute differences, and how much is reactor specific, but sampling gas representative of that incident on the substrate surface is obviously preferable from the viewpoint of any detailed understanding the CVD process. We now wish to build on these lessons and develop a new, more versatile, and much more sensitive, molecular beam mass spectrometer (MBMS) experiment. The proposed experiment is based on a microwave reactor, designed to be as similar as possible to that recently provided by Element Six Ltd for laser diagnosis. It will be equipped with a small (<100 μm diameter), carefully designed, orifice in the substrate centre. Process gas exiting through this orifice will pass into a differentially pumped first stage (p <10-4 Torr) in the form of a supersonic molecular beam, through a carefully aligned skimmer cone, into a second chamber (p <10-6 Torr), through a rotating chopper wheel (to allow use of phase sensitive detection methods and thereby enhancing the contrast between beam and background gas molecules), and then into the source region of a differentially-pumped QMS system equipped with a variable energy electron impact ionisation source. This will offer the opportunity to detect radical species free from interferences from the (generally much more abundant) stable molecular species, since the former can be formed by ionisation at much lower impact energies than would be needed to produce the corresponding ions by dissociative ionisation of a stable parent molecule. A small positive bias at the entrance to the ionisation region will ensure rejection of any ions transported from the plasma; study of this ionised component should also be possible, using the QMS with the ioniser ‘off’. A schematic diagram of the planned apparatus is shown in Fig.1, which also indicates potential suppliers of the various parts, some of which can be recycled from the existing MBMS experiment. Given our ever-growing use of laser diagnostic methods, the MW reactor will be designed with sufficient optical access to allow the possibility of making line-of-sight column density measurements (at least) close to the substrate surface. What new science will an improved reactor-MBMS system enable? For the first time, we will be sampling process gas at the surface of the growing diamond film in a MW-PECVD reactor – which has been designed to approach industry standards, and to match the existing (laser diagnosed) reactor thereby encouraging complementary and transferability of trends in data measured with the two set-ups. We anticipate a >103 sensitivity gain compared to our previous apparatus, as a result of careful consideration of orifice sizes, propagation distances in the various pressure regions, much improved pumping speeds, and the incorporation of a properly-designed chopper. Just in the context of the basic CH4/H2 (and CH4/Ar/H2) gas mixtures, this should afford us the opportunity to make the first careful studies of the relative (and absolute) abundances of reactive species like C, CH x, C2, C2Hx, C3, C3Hx – in parallel, and as a function of process conditions. The plasma chemical model and thermochemical reaction mechanism that we have developed thus far, and which has been used in all of our most recent comparisons with experiment, still has a number of recognised limitations – which can be tested, validated or improved, as necessary, by experiments of this kind. The plasma jet is referred to as a transferred arc. The Ar/H2 mixture exiting the twin torch nozzles comprises H and Ar atoms, Ar + ions and electrons; this reacts with background H2 and with hydrocarbon introduced down stream. By the time this plasma reaches the growing film surface 14 cm downstream, the plasma is essentially thermal and the ion and electron densities are low; experimental measurements close to the substrate thus do not offer a particularly stringent test of the ion chemistry currently in the model. Conversely, in a MW reactor the centre of the plasma ball – the region of highest electron impact excitation probability, where carbon containing ion densities will be maximal – is only mm from the substrate. Model refinement requires direct measurements of regions where gas phase ion chemistry makes a significant contribution to the overall hydrocarbon processing; the proposed in situ MBMS measurements offer what currently looks to be the best route to such data. The current (predominantly neutral) reaction mechanism terminates with C 4H2 as the heaviest hydrocarbon species. Given lack of any detailed information to the contrary, we treat this as a ‘bin’ for any and all heavier hydrocarbon species in the plasma. Clearly, the proposed MBMS sampling facility will enable a much more detailed investigation of the number and nature of the heavier hydrocarbons in such plasmas, and the way their distribution varies with input hydrocarbon mole fraction. Such carbon condensation reactions are expected to become progressively more important as the input hydrocarbon/H2 ratio increases and the deposited material evolves from microcrystalline diamond through nanocrystalline diamond (NCD) to ultrananocrystalline diamond (UNCD) – a topic to which we return below. Another possible variable – time – is largely unexplored in the context of the gas phase inter-conversions accompanying diamond CVD, but time-dependent studies could offer further stringent tests of the modelling. The number and column density measurements we have published thus far have all been taken under ‘steady-state’ conditions, but the QC laser column density measurements of hydrocarbon/H2/Ar gas mixtures in the MW reactor made during the past summer have allowed study of the build-up and decay of individual species (CH4, ground state and vibrationally excited C2H2 in this case), on a sub-second timescale, after switch on (or off) of the hydrocarbon feed gas flow. These studies have revealed dramatic, and quite unexpected, species-specific time dependences, the interpretation of which is complicated by the averaging implicit in any line-of-sight (rather than point) measurement. The QMS can also be massscanned on this time-scale; the through substrate-sampling scheme will allow the opportunity of sampling the timedependent build-up and decay of a wide range of species concentrations incident on the substrate surface, simultaneously. 5 Such measurements would not only provide further stringent tests of the existing reactor modelling but also guide existing efforts to deposit extremely thin multilayer structures (e.g. of insulating and semiconducting diamond). The potential opportunities of the proposed MBMS sampling of a purpose designed MW-PECVD reactor appear even more wide-ranging when we consider environments for doped diamond growth. Our current work of B2H6/CH4/H2 gas mixtures [19] has revealed an almost total absence of data on B/C/H coupling reactions. In what form is boron evacuated from a CVD reactor? What about nitrogen? HCN is the thermodynamically favoured stable product (as with HCCH in activated CH4/H2 gas mixtures), but what are the most abundant reactive N-containing species adjacent to the growing diamond surface? CN? Methylene imine (CH 2NH)? The answers to such questions will be essential prerequisites for guiding the next generation of computational studies of diamond surface growth from the gas phase – both for hydrocarbon/H2 gas mixtures [24] and for doped growth (e.g. B doped diamond [25]). The new apparatus should be equally capable of providing new insights into the growth mechanisms of nanocrystalline diamond (NCD) and ultrananocrystalline (UNCD) films. NCD films can be grown simply by increasing the concentration of hydrocarbon present within the gas mixture during growth, from the usual 1% (typically used to deposit high quality microcrystalline films) to 5-10% [26]. This causes the average grain size to shrink from microns to ~50 nm; the resulting NCD films have been termed ‘cauliflower’ or ballas diamond. Indeed, we demonstrated growth of such films way back in the early 1990s. UNCD film growth has been pioneered at Argonne National Laboratory [27-29]. The films are grown from a rather different gas chemistry involving a few %CH 4 in Ar, with little additional H2 and pressures 100-200 Torr (i.e. somewhat greater than that typically used in microcrystalline diamond growth). These MW activated CH4/Ar/H2 mixtures yield diamond films with grain sizes of <5 nm, which are very smooth and uniform. They contain far fewer graphitic inclusions than NCD films; analysis shows them to consist of nanodiamond crystallites with atomically abrupt grain boundaries. The largely -bonded grain boundaries, which actually contain around 10% of the carbon in the films, contain a plethora of states that partially fill the diamond band-gap – which has important consequences for the mechanical, electrical and optical properties of the material. Many of these properties appear to be tuneable by doping impurities, e.g. nitrogen, into the films. UNCD is also starting to find a range of niche applications, e.g.: micro-electromechanical systems (MEMS), rotating shaft pump seals, photonic switches, electronic devices (p-n junctions), field emission cathodes, electrochemical electrodes and biosensors, and hermetic coatings on bio-implants [30]. Although this material is attracting much excitement, there have, as yet, been few studies on the fundamental chemistry of its growth. The Argonne group have made simple optical emission spectroscopy (OES) studies of the light from the CH4/Ar/H2 plasma, and identified intense emissions from excited C2 radicals and little H atom emission. This has led to the suggestion [28] that the UNCD film growth mechanism is very different to that for normal microcrystalline diamond films, in which the surface chemistry is driven by atomic H abstraction reactions and step-wise addition of CH3 radicals. In contrast, for UNCD it has been proposed that growth occurs by direct insertion of the C 2 radical into the diamond surface, followed by rearrangement. OES is an unsophisticated plasma diagnostic technique, however, the results of which are notoriously prone to misinterpretation since it is insensitive to ground state (usually the majority) species, and to many species which do not emit at all, even when in excited states. Even for those that do emit, the method generally does not yield accurate species concentrations. Recent work by ourselves [31,32], the Heriot-Watt group [33] and other groups at Argonne [34] have cast doubt on the C2 growth mechanism; our combined experiment and modelling analyses thus far indicate that methyl, C2H and/or atomic C are all likely to be more important growth species. The proposed MBMS apparatus, with its through-substrate orifice specifically-designed to sample from higher pressure (<200 Torr), hot plasmas, will be ideally suited to investigating this issue. Allied with companion modelling it should be possible to resolve this mechanistic controversy. Doping (particularly nitrogen doping) of UNCD films is another area of considerable contemporary interest – not least because of their reported n-type semiconductivity of films grown from N2/CH4/H2 gas mixtures but, again, the only gas phase chemistry studies reported to date are simple OES experiments [35]. Clearly, the proposed MBMS system will allow measurement of the concentrations of the major species in the plasma, and thereby help pin-down more accurately the role played by nitrogen. 6 Figure 1 Programme of Work The necessary MW power supply and waveguide, tuners, etc are already available from our existing apparatus, as are most of the necessary gas delivery manifolds and pressure gauges. A new deposition chamber is required, which will couple to existing MW power supply at the top, and to the MBMS system below as illustrated in Fig.1. The reactor will be designed in very close collaboration with Dr John Brandon (Element Six Ltd) – who was responsible for the design of the recently-supplied MW reactor optimised for laser diagnosis – so as to ensure comparability between data taken in the two reactors. The sampling orifice in the new reactor has to be carefully designed into the substrate platen itself. Design of this stage, in particular ensuring adequate heat dissipation from the region of the <100 m sampling orifice, has been a particular challenge. The favoured design, which will employ (replaceable) Mo apertures gold-brazed into a Mo platen, has been guided by a recently-reported substrate assembly design that has been used for successful MBMS sampling of flames [37]. As Fig.1 shows, the MBMS system will involve a total of three stages of differential pumping (the two-stage design adopted in our first generation MBMS, and the inadequate pumping speed employed in the first stage, were the biggest limitations of that system). Other design features of note are the skimmer mounts, which will be designed to allow careful skimmer alignment with a laser beam prior to their being locked down in position, the chopper (to allow phasesensitive detection with the QMS and thus discriminate against the inevitable background hydrocarbon signals in any mass spectrometer system) and the fact that the QMS assembly will be translatable (recycling an existing translation bellows assembly) so as to position its electron impact source region optimally around the beam of sampled gas. The proposed schedule of work will be sequenced very much as the scientific description in part A. The PDRA will be appointed at the outset, and be responsible for system construction, validation, and first proving measurements. All being well, the PhD student will join the project after 6 months, and progressively gain knowledge and expertise by working alongside the PDRA so that he/she is able to take lead responsibility for operating the experiment when the PDRA leaves. Such an arrangement worked extremely well when establishing the original MBMS system in 1994. Dilute hydrocarbon/H2 gas mixtures, such as those used for microcrystalline CVD diamond film growth, have already been studied extensively, by ourselves and others, but questions remain. Initial experiments will focus on such mixtures – to prove that the new MBMS system is behaving properly and yielding trustworthy results, to establish the optimal electron impact conditions for detecting the various radicals of interest (CH3, C2H, C, etc.) and, in conjunction with the modelling, to estimate sampling efficiencies and detection limits for these various species and characterise as fully as possible the new MW reactor, its flow fields, gas temperature and power density distributions, etc., as a function of process conditions. Naturally, we will seek to correlate gas phase species concentrations and distributions measured by the MBMS with results from our on-going, complementary, laser diagnostic studies, with model predictions, and with properties of material grown under the various process conditions. The focus of attention will then move on to more exotic mixtures – both doped dilute hydrocarbon/H2 mixtures (e.g. B2H6/CH4/H2) and the more concentrated hydrocarbon/Ar/H2 mixtures used in (U)NCD growth as outlined in A 7 above and in the timeline shown in the Appendix. The latter, in particular, will present new challenges. Sampling and transport through the skimmer is significantly different when using a heavier majority gas (Ar or N 2) in place of H2 – issues with which we have some familiarity given the earlier sampling of MW plasma activated CO 2/CH4 gas mixtures. The rewards could be considerable, however – not just in terms of resolving existing controversies about (U)NCD growth mechanisms, but also in terms of the new insights such studies could provide to our understanding of carbon nucleation (the trigger for most soot formation. Personnel: 1 year PDRA funding and a 3-year PhD project studentship are required. The PDRA will initiate the project, assemble the new MBMS system, and be responsible for the proving experiments. The student will progressively assume responsibility for the experiment, and be in a position to run it, and to harvest and analyse data single-handedly, by the time the PDRA’s contract expires. The student will also have the opportunity to receive training on, and to perform, ex situ film characterisation, (e.g. laser Raman, SEM and field emission experiments), as well as assist with some of the aforementioned surface analysis. The project is very interdisciplinary, and the student will gain valuable experience in a wide range of contemporary and relevant areas of science, including vacuum technology, plasma diagnosis, thin film deposition, gas phase modelling, surface analysis, as well as transferable skills such as I.T. and the personal skills required to liaise successfully between a number of related groups within the School of Chemistry and related departments, such as Physics and the IAC. Past students involved in such work have progressed to employment in high tech industries such as AWRE and Renishaw Ltd. References 1 P.W. May, Phil. Trans. R. Soc. Lond. A, 358 (2000) 473. 2 M.N.R. Ashfold, P.W. May, J.R. Petherbridge, K.N. Rosser, J.A. Smith, Y.A. Mankelevich, N.V. Suetin, Phys.Chem.Chem.Phys., 3 (2001) 3471. 3 C.A. Rego, P.W. May, M.N.R. Ashfold, et al., Diam. Rel. Mater. 4 (1995) 770, J. Appl. Phys. 79 (1996) 7264, Diam. Rel. Mater. 7 (1998) 1651. 4 S.M. Leeds, P.W. May, M.N.R. Ashfold, et al Diam. Rel. Mater. 8 (1999), 226, 1377. 5 J.R. Petherbridge, P.W. May, M.N.R. Ashfold, et al., J. Appl. Phys, 89 (2001) 1484, Diam. Rel. Mater, 10 (2001) 393. 6 J.R. Petherbridge, P.W. May, M.N.R. Ashfold, et al. Diam. Rel. Mater. 11 (2002) 301, Phys. Chem. Chem. Phys. 4 (2002) 5199. 7 S.A. Redman, C. Chung, M.N.R. Ashfold, et al., Diam. Rel. Mater. 8 (1999) 1383, Phys. Chem. Chem. Phys. 1 (1999) 1415. 8 J.A. Smith, E. Cameron, M.N.R. Ashfold, Y.A. Mankelevich and N.V. Suetin, Diam. Rel. Mater. 10 (2001) 358, 364. 9 J.A. Smith, K.N. Rosser, H. Yagi, M.I. Wallace, P.W. May and M.N.R. Ashfold, Diam. Rel. Mater. 10 (2001) 370. 10 M.D. Wheeler, S.M. Newman, A.J. Orr-Ewing and M.N.R. Ashfold, J. Chem. Soc. Faraday Trans., 94 (1998) 337. 11 J.B. Wills, J.A. Smith, W.E. Boxford, J.M.F. Elks, M.N.R. Ashfold and A.J. Orr-Ewing, J. Appl. Phys. 92 (2002) 4123. 12 C.J. Rennick, M.N.R. Ashfold, A.J. Orr-Ewing, Yu.A. Mankelevich et al., Chem. Phys. Lett. 383 (2004), 518, Diam. Rel. Mater. 13 (2004) 561. 13 C.J. Rennick, R. Engeln, J.A. Smith, A.J. Orr-Ewing, M.N.R. Ashfold and Yu.A. Mankelevich, J. Appl. Phys. 97 (2005) 113306:1-15. 14 See, for example, W.N. Wang, N.A. Fox, P.W. May, et al., Phys. Stat. Sol., 154 (1996) 255. 15 K.E. Spear and J.P. Dismukes, Synthetic Diamond - Emerging CVD Science and Technology (Wiley, New York, 1994). 16 M.H. Nazare and A.J. Neves (eds.) Properties, Growth and Applications of Diamond (INSPEC 2001). 17 J.B. Cui, J. Ristein, and L. Ley, Phys. Rev. Lett. 81 (1998) 429. 18 Diamond for Electronic Applications eds. D.L. Dreifus, A. Collins, T. Humphreys, K. Das, P. Pehrsson (MRS Symp. Proc.) Vol 416 (1996). 19 D.W. Comerford, A. Cheesman, …, M.N.R. Ashfold and Yu.A. Mankelevich, J. Phys. Chem. A (in press). 20 A. Deneuville, Semiconductors and Semimetals, 76 (2003) 183 and references therein. 21 J. Angus, Y.V. Pleskov and S.C. Eaton, Semiconductors and Semimetals, 77 (2004) 97 and references therein. 22 C.A. Rego et al., in 'Advances in New Diamond Science and Technology' (eds. S. Saito et al.), MYU, Tokyo, 1994, pp. 485. 23 A. Kováts and P. Deák, Diam. Relat. Maters. 14 (2005) 1517. 24 See, for example, J.K. Kang and C.B. Musgrave, J. Chem. Phys. 113 (2000) 7582. 25 A. Cheesman, J.N. Harvey and M.N.R. Ashfold, Phys. Chem. Chem. Phys. 7 (2005) 1121. 26 For SEM images of NCD films grown in Bristol see: M.N.R. Ashfold, P.W. May, C.A. Rego, N.M. Everitt, Chem. Soc. Rev., 23 (1994) 21. 27 D.M. Gruen, S. Liu, A.R. Krauss, J. Luo, and X. Pan, Appl. Phys. Lett. 64, (1994) 1502. 28 D. Zhou, T.G. McCauley, L.C. Qin, A.R. Krauss, and D.M. Gruen, J. Appl. Phys. 83, (1998) 540. 29 A.R. Krauss, O. Auciello, et al., J. Appl. Phys. 89 (2001) 2958, and references therein. 30 http://chemistry.anl.gov/MSD/PDF/MRS_Metal.pdf 31 E. Crichton, P.W. May, unpublished results presented at ICNDST-9, Tokyo, April 2004, and Diamond 2004, Italy. 32 P.W. May, Yu. Mankelevich and J.A. Smith, Diam. Relat. Maters. (2005), in press. 33 P. John, J. Rabeau and J.I.B. Wilson, Diam. Rel. Mater. 11 (2002) 608. 34 X. Xiao, J. Birrell, J.E. Gerbi, O. Auciello and J.A. Carlisle, J. Appl. Phys. 96 (2004) 2232. 35 T.D. Corrigan, D.M. Gruen, A.R. Krauss, P. Zapol and R.P.H. Chang, Diam. Relat. Maters, 11 (2002) 43. 37 S. Park, F. Liao, J.M. Larson et al., Plas. Chem. Plas. Proc. 24 (2004) 353; http://www.me.umn.edu/~mechpark/research.html