Template for Electronic Submission to ACS Journals
advertisement
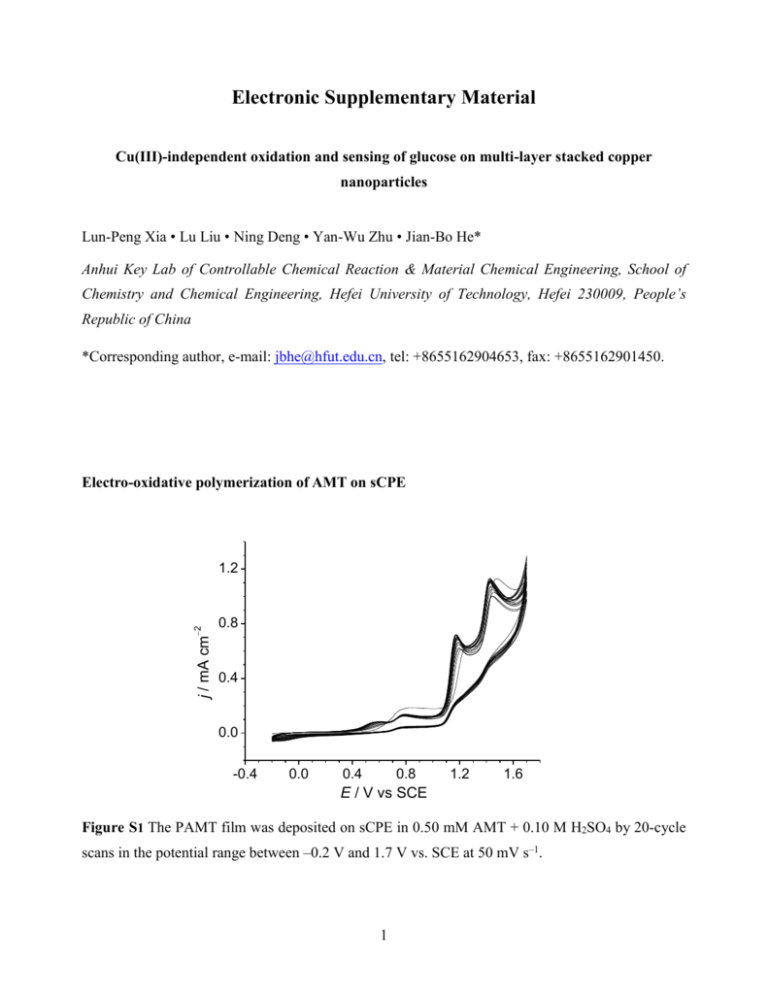
Electronic Supplementary Material Cu(III)-independent oxidation and sensing of glucose on multi-layer stacked copper nanoparticles Lun-Peng Xia • Lu Liu • Ning Deng • Yan-Wu Zhu • Jian-Bo He* Anhui Key Lab of Controllable Chemical Reaction & Material Chemical Engineering, School of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei 230009, People’s Republic of China *Corresponding author, e-mail: jbhe@hfut.edu.cn, tel: +8655162904653, fax: +8655162901450. Electro-oxidative polymerization of AMT on sCPE j / mA cm 2 1.2 0.8 0.4 0.0 -0.4 0.0 0.4 0.8 1.2 1.6 E / V vs SCE Figure S1 The PAMT film was deposited on sCPE in 0.50 mM AMT + 0.10 M H2SO4 by 20-cycle scans in the potential range between –0.2 V and 1.7 V vs. SCE at 50 mV s–1. 1 Experimental data with only Cu on CPE for comparison with Cu/PAMT/sCPE Figure S2 FE-SEM images of Cu/PAMT/sCPE (A and B) and Cu/sCPE (C and D) at a magnification of ×10.0k (A and C) and of ×100k (B and D). The PAMT film was deposited on sCPE in 0.50 mM AMT + 0.10 M H2SO4 by CV scan between –0.2 V and 1.7 V vs SCE at 50 mV s–1 for 20 cycles. The Cu particles were deposited from 0.010 M CuSO4 + 0.50 M H2SO4 at –0.80 V vs SCE for 10 min. The Cu particles on the PAMT inner layer show a larger nucleation density and a smaller individual size than the ones deposited directly on the sCPE. 2 12.0 j / mA cm 2 glucose a 8.0 b 4.0 b' a' 0.0 -0.2 0.0 0.2 0.4 0.6 0.8 E / V vs SCE Figure S3 CV curves of Cu/PAMT/sCPE (a and a) and Cu/sCPE (b and b) in 0.10 M NaOH with (a and b) and without (a and b) 0.20 mM glucose. Scan rate 50 mV s–1. The electrodes were prepared under the same conditions as in Figure S2. 0.5 0.4 j / mA cm 2 III 0.3 IV I e d II 0.2 II' c b 0.1 a -0.2 0.0 0.2 0.4 0.6 0.8 E / V vs SCE Figure S4 DPV responses of Cu/sCPE to glucose. The experimental conditions were exactly same as those with Cu/PAMT/sCPE in Fig. 2b. 3 j / mA cm 2 10 8 6 4 2 0 400 500 600 700 t/s Figure S5 Amperometric response of Cu/sCPE upon successive addition of glucose. The experimental conditions were exactly same as those with Cu/PAMT/sCPE in Fig. 4. Electrochemical mechanism of glucose oxidation at Cu/PAMT/sCPE The mediator for two-electron oxidation of glucose in the absence of Cu(III) needs careful experimental verification. Some reactive O-containing species are possible candidates. The hydroxyl radical produced and absorbed on electrode surfaces has been well known to be of catalytic importance in small organic compound oxidation. It has been considered as the redox mediator for the oxidation of carbohydrates and other organic molecules on Cu2O/C-paste electrodes in 0.1 M NaOH, although the standard potential for •OH/OH– was reported as +1.33 V vs. Ag/AgC1 [1]. Another possible O-containing mediator is the surplus O2– ions within the cupric oxide layer, which come from the decomposition of OH–/H2O under the strong electric field across the electrode|solution interface, as detailed discussed in our previous work [2]. The oxide layer on Cu showed unusually high capacitances, which indicated that the surplus charge density in the space-charge region was high enough to cause surface degeneracy. During the potential scan, the capacitance gradually reached a maximum near the onset potential of oxygen evolution and then decreased with increasing current from the oxidation of O2– to O2. Zhuravlyova et al. [3] demonstrated that O2 could be generated within anodic films on Al–Cu alloys by oxidation of O2– ions, and was present in numerous bubbles in the bulk film. Oxygen was also present in alumina anodic films but in low amounts. The highly active O2– ions may possibly be involved in the 4 oxidation of the adsorbed glucose to form gluconolactone and/or gluconate in alkaline media, through a two-electron reaction as below: O | | HOCH 2CH(CHOH)3 (CHOH) + O 2 O | | HOCH 2CH(CHOH)3CO + H 2O + 2e OH HOCH 2 (CHOH) 4COO Long-term stability of electrodes Relative response / % 110 100 a 90 b 80 70 t / day 60 0 10 20 30 40 50 60 Figure S6 Relative amperometric responses of Cu/PAMT/sCPE (a) and Cu/sCPE (b) in 0.10 M NaOH containing 0.10 mM glucose, over a period of 57 days. Applied potential, 0.50 V vs. SCE References 1. 2. 3. Xie Y, Huber CO (1991) Electrocatalysis and amperometric detection using an electrode made of copper oxide and carbon paste. Anal Chem 63:1714-1719 He J-B, Lu D-Y, Jin G-P (2006) Potential dependence of cuprous/cupric duplex film growth on copper electrode in alkaline media. Appl Surf Sci 253:689-697 Zhuravlyova E, Iglesias-Rubianes L, Pakes A, Skeldon P, Thompson GE, Zhou X, Quance T, Graham MJ, Habazaki H, Shimizu K (2002) Oxygen evolution within barrier oxide films. Corros Sci 44:2153-2159 5