The key role of meiofaunal nematodes in coastal wetlands
advertisement
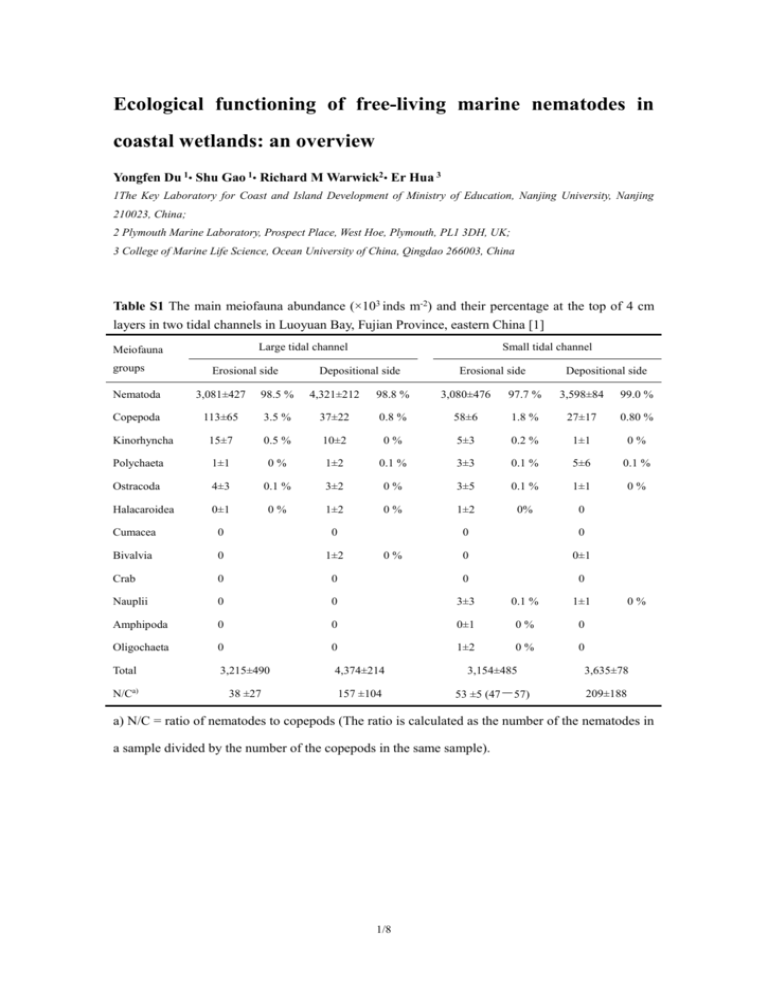
Ecological functioning of free-living marine nematodes in coastal wetlands: an overview Yongfen Du 1• Shu Gao 1• Richard M Warwick2• Er Hua 3 1The Key Laboratory for Coast and Island Development of Ministry of Education, Nanjing University, Nanjing 210023, China; 2 Plymouth Marine Laboratory, Prospect Place, West Hoe, Plymouth, PL1 3DH, UK; 3 College of Marine Life Science, Ocean University of China, Qingdao 266003, China Table S1 The main meiofauna abundance (×103 inds m-2) and their percentage at the top of 4 cm layers in two tidal channels in Luoyuan Bay, Fujian Province, eastern China [1] Large tidal channel Meiofauna groups Erosional side Small tidal channel Depositional side Erosional side Depositional side Nematoda 3,081±427 98.5 % 4,321±212 98.8 % 3,080±476 97.7 % 3,598±84 99.0 % Copepoda 113±65 3.5 % 37±22 0.8 % 58±6 1.8 % 27±17 0.80 % Kinorhyncha 15±7 0.5 % 10±2 0% 5±3 0.2 % 1±1 0% Polychaeta 1±1 0% 1±2 0.1 % 3±3 0.1 % 5±6 0.1 % Ostracoda 4±3 0.1 % 3±2 0% 3±5 0.1 % 1±1 0% Halacaroidea 0±1 0% 1±2 0% 1±2 0% 0 Cumacea 0 0 0 0 Bivalvia 0 1±2 0 0±1 Crab 0 0 0 0 Nauplii 0 0 3±3 0.1 % 1±1 Amphipoda 0 0 0±1 0% 0 Oligochaeta 0 0 1±2 0% 0 Total 3,215±490 4,374±214 3,154±485 3,635±78 N/Ca) 38 ±27 157 ±104 53 ±5 (47-57) 209±188 0% 0% a) N/C = ratio of nematodes to copepods (The ratio is calculated as the number of the nematodes in a sample divided by the number of the copepods in the same sample). 1/8 Table S2 Some representative investigations carried out in China seas Sampling area Bohai Sea (Yellow River estuary) Sampling date The Yellow Sea (YS) The East China Sea The South China Sea (Beibu gulf) Shelf waters in the southern of YS Subtidal area in the southern of YS The whole area Changjiang River estuary Taiwan Strait Jul.-Aug. 1986 Jan. 2003 June 2003 Jul. 2008 Jun. 2003 & Sep. 2004 Aug.1997; Feb. 1998 Jul.2006, Jan., Apr. & Oct. 2007 Abu. a) 527-1,300 831±247 1,404±670 1,193 ± 689 1,785±496 - ~500-1,000 Species num. (- ) 223 (145 genera) 232 (149 genera) 294 (110 genera) 263 (119 genera) 85-100 (75-91 genera ) (102 genera) Dominant species b) The subaquatic delta: Dorylaimopsis sp., Retrotheristus sp., Parodontophora sp. Dichromadora sp. Subtidal coldwater community in muddy habitat: Prochromadorella attennuata, Dorylaimopsis rabalaisi, Metalinhomoeus longicauda, Sphaenolaimus minitus, Paramonohystera sp. & Campylaimus gerlachi Shandong Coast: Microlaimus sp., Parasphaerolaimus sp., Leptolaimus sp., Halalaimus gracilis & Dorylaimopsis. rabalaisi; The southern YS: Genus Dorylaimopsis, Sphaerolaimus, Sabatieria, Parodontophora & Daptonema Estuarine brackish: Genus Halalaimus, Daptonema & Metalinhomoeus Jiangsu coast: Daptonema sp., Ptycholaimellus sp., Prochromadorella sp., & Chromadorita sp. The northern YS: Genus Sabatieria, Parodontophora, Chromadora , Neochromadora, Sphaerolaimus & Dorylaimopsis; The northern YSCWM: Parodontophora Central & northern: Sabatieria, sp., Dorylaimopsis variabilis, Vasostoma sp., Spilophorella sp., Daptonema sp., Linhystera sp., Halalainmus sp., & Filoncholaimus sp. The Southern: Dorylaimopsis Sabatieria sp., Vasostoma sp. Genus Dorylaimopsis, Sabatieria, Sphaerolaimus, Parodontophora, Elzalia, Terschellingia, Metadesmolaimus, Setosabatieria & Paracomesoma the Laizhou Bay: Parodontophora sp., Dorylaimopsis sp., Paralinhomoeus sp., Spilophorella sp. & Sphaerolaimus sp. The central part of Bohai: Yellow Sea Cold Water Mass (YSCWM): Paramonohystera sp., Prochromadorella sp., 2/8 Oxygen minimum zone: Genus Daptonema, Cobbia & hypoxia tolerant Sabatieria. Continental shelf shallow water on the Southern of the Hangzhou Spilophorella sp., Dichromadora sp. & Sabatieria sp. Promonhystera sp., D. rabalaisi & Ptycholaimellus sp. Transitory community: Microlaimus sp., D. rabalaisi., Cobbia sp., Aegialoalaimus sp. & Leptolaimus sp. marina, Sabatieria sp., Comesa sp., Parodontophora sp. & Chromadora sp. Bay: Genus Cobbia, Terschellingia. & Leptolaimus & Xyala sp. - 2A (34 %), 1A (30 %) 1B (28 %) 2B (8 %) 2A (31 %) 1B (31 %), 1A (29 %) 2B (10 %) 2B (36 %) 1B (30 %), 1A (19 %) 2A (14 %) 2A (35 %) 1B (33 %) 1A (27 %) 2B (5 %) 2A (31-38 %) 1A (29-38 %), 1B (25-26 %) 2B (8-14 %) 2A (35 %) 1B (30 %), 2B (13 %) 1A (10 %) Main regulators Sedimentation rate, food availability & larval complement Organic matter, clay content & Chlorophyll-a Water depth, organic matter & sediment water content Phaeopigment & organic matter Chlorophyll-a, phaeopigment & sediment grain Sediment composition & sea current Water depth & human disturbance Reference [2, 3] [6] [7] [8, 9] [10, 11] [12] Trophic groups c) [4, 5] a) The unit of abundance is ×103 inds m-2; - no data are given; b) arranged in decreased order of their contribution to total abundance; c) Wieser’s classification [13], namely 1A = selective-deposit feeders), 1B = non-selective deposit feeders, 2A = epigrowth feeders, 2B = predators/omnivores. 3/8 Table S3 Community structure of marine nematodes in coastal wetland in China Sampling area & vegetation Sandy-beach Cordgrass Mangrove wetland Organically-polluted flat (Qingdao Bay) SA, SM & PA (Yangtze River estuary) KC (Shenzhen Bay) Jun. & Oct. 1990 Mar. & Aug. 2004 Jan., Apr., Jul. & Oct. 1997 Abundance (×103 inds m-2) ~3,400 (~15-16,000) SA: ~100-600 SM: ~100-700 PA: ~100-1,000 a) ~ 300-800 Species num. 20 (18 genera) - (52 genus, ~15-20 genera each plant ) 28 Dominant species Metoncholaimus moles, Paracanthonchus monodons, Innocuonema sp., Paramonohystera pellnerda, Sabatieria pulchra, Desmolaimus zeylandicus & Theristus metaflevensis SA: Genus Terschellingia, Ethmolaimus, Parodontophora, Desmoscolex, Daptonema, Leptolaimus, & Halalaimus SM: Polysigma, Theristus, Daptonema, Parodontophora, Hypodontolaimus, Terchellingia & Anoplostoma PA: Genus Haliplectus, Dilochodorus,Hypodontola imus & Parodontophora Sampling date Bared flat (Tong’an Bay) Bared flat (Jiulong estuary) Feb. & May 1999 Dec. 1997, Mar., May, Jun., Jul. 1998 KC: ~120 AM: ~200-1,500 ~ 200-400 ~1,610-2,670 48 (41 genera) - (-) (22 genera) 37, 21-23 each type (20-22 genera each type) Terschellingia sp., Daptonema sp., Metalinhomoeus sp., Parodontophora sp., Sabatieria sp., Eumorpholaimus sp., & Microlaimus sp. KC: Sabatieria sp., Terchellingia sp., Viscosia sp., Terschellingia sp., Elzalia sp. & Parodontophora sp. AM: Chromadorina sp., Eumorpholaimus sp., Ethmolatmus sp., Terchellingia sp. & Spilophorella sp. Dorylaimopsis variable, Viscosia sp., Daptonema sp., Sphaerolaimus balticus, & Thalassomonhy stera sp. Daptonema sp., Eumorpholaimus sp., Terschellingia longicaudata, Paramonohystera sp., Sphaerolaimus sp., Leptolaimus sp., Dorylaimus sp., Microlaimus sp. & Monhysterides sp. 4/8 AM & KC (Tong’an Bay) Muddy flat Dec. 2004 Dominant Feeder b) 2B & 2A Bacteria & algal feeder c) - KC: 1B, 1A, 2 A AM: 2A &1B Main regulators - Plant type - Organic matter Reference [14] [15] [16] [17] - 1B &1A Food resource Organic matter & sediment composition [18] [19] a) Unit: inds 10 g dry sediment; b) Wieser’s classification [13] (1A = selective-deposit feeders, 1B = non-selective deposit feeders, 2A = epigrowth feeders, 2B = predators/omnivores); c) according to [20]: 6 trophic groups (algal feeder, plant feeder, bacterial feeder, fungi feeder, carnivore feeder & Omnivore feeder). SN= Scripus mariqueter; PA= Phragmites australis; SA= Spartina alterniflora, AM= Avicennia marina ; Kandelia candel= KC -1 5/8 Table S4 Research priorities for the marine nematodes in the near future Research themes Scientific questions and objectives Global distribution patterns Fundamental descriptive work Finding out the key regulators and their patterns of spatial and temporal change Vulnerability and adaptation of nematodes to disturbance Identifying individual species and the species composition of samples quickly Whether specific species exist in particular habitat types (e.g. seagrass, salt-marsh or tidal flat) Being cosmopolitan (as protists) or a restricted geographical distribution (as large animals) Nematodes predation on microbenthos Contribution of nematodes to microbial food web Macrofaunal predation on nematodes Bottom up control of nematodes as a food resource for macrofauna Succession patterns in different salt-marshes or regional geological and geographic conditions Elucidating the processes and mechanisms involved in different ecosystems Offering a theoretical basis for further management Roles in pelagic-benthic coupling Contribution to geochemical cycles: a carbon “sink” or “source” Environment monitoring Laboratory and mesocosm experiments for typical nematodes or communities Long-term monitoring in typical regions Integrated analysis using computer-based data Responses to pollutants, global change (e.g., acidity of seawater, greenhouse effects, rising sea level) Accumulating a database for model construction or other basic research Establishing evaluation criteria widely-accepted not only recognized by specialists Applied contexts Nutritional analysis Nutritional value of individuals that utilize different foods or at different life stages Finding out the potential feasibility of mass culture as a food source for aquaculture Test their possible application in medical research or other industries (e.g., neurotoxins, cellulases and polyunsaturated fatty acids, amino acid synthesis) Biodiversity assessments Ecosystem dynamics in salt-marshes Diversity descriptions in diverse habitats Diversity drivers Tolerance to disturbances DNA barcoding Characteristics of specific habitats Characteristics of substances produced by certain nematodes Methology Improved approaches for nematodes’ extraction and enumeration Mathematical models Quick and accurate estimation of the abundance and biomass for a large number of samples Predicting succession patterns of particular ecosystems in response to disturbance 6/8 Computerization of databases Including DNA sequences, taxonomy, phylogeny, morphology (including photographs), ecology, socio-economic value and literature Offering a comprehensive platform for research, socio-economic issues, general awareness and accessibility of the public 7/8 References 1. Du YF (2012) Response of benthos commnunity to the introduction of exotic Spartina alterniflora: a case study from salt-marshes in Luoyuan Bay, Fujian and Northern Jiangsu. Postdoctor Report, Nanjing University 2. Zhang ZN, Li YG, Tu LH et al (1989) Preliminary study on the ecology of the benthic meiofauna in the Huanghe River estuary and its adjacent waters. Oceanol Limnol Sin 3: 197–208 (in Chinese) 3. Zhang ZN, Gu F, Yu ZS (1990) A study on spatial pattern of marine nematodes in the subaqueous delta of the Huanghe River. Oceanol limnol Sin 21: 11–19 (in Chinese) 4. Huang Y, Zhang ZN, Liu XS et al (2006) Studies on the species composition and biodiversity of free-living marine nematodes in the southern Huanghai Sea. Acta Oceanol Sin 25: 87–98 (in Chinese) 5. Huang Y, Zhang ZN, Liu XS (2007)The marine nematodes community in winter Southern Yellow sea. Oceanol Limnol Sin 38: 199–205 (in Chinese) 6. Liu XS, Zhang ZN, Huang Y (2007) Sublittoral meiofauna with particular reference to nematodes in the Southern Yellow Sea, China. Estuar Coast Shelf Sci 71: 616–628 7. Wu XQ (2011) Community structure and distribution of meiofauna in the Yellow Sea and East China Sea in summer. Dissertation, Ocean University of China 8. Hua E, Zhang ZN, Yu ZS et al (2010) Preliminary study on the immediate response of the nematode community to typhoon soudelor. Deep-Sea Res Part II: Topical Studies in Oceanography 57: 1064–1070 9. Hua E, Zhang ZN, Zhang Y (2005) Abundance and biomass of meiobenthos in the Changjiang (Yangtze river) estuary and its adjacent waters. Acta Ecol Sin 25: 2234– 2242 (in Chinese) 10. Cai LZ, Hong HS, Zou CZ et al (2000) Studies on species composition of marine nematodes and their food types in south Taiwan strait. J Oceanogr Taiwan Strait 19: 212– 217(in Chinese) 11. Cai LZ, Hong HS, Zou CZ et al (2001) Species composition and distribution of marine nematode community in the north Taiwan strait. Acta Oceanol Sin 20: 221– 229 (in Chinese) 12. Fu SJ, Cai LZ, Yang J et al ( 2012) Spatial and seasonal variations of subtidal free-living nematode assemblages in the northern Beibu gulf, South China Sea. J Mar Biol Ass UK 92: 255–264 13. Wieser W (1953) Die beziehung zwischen mundhohlengestalt, ernahrungsweise und vorkommen bei freilebenden marinen nematoden. Arch Zool 2: 439–484 14. Zhang ZN, Dang HY, Yu ZS (1993) Studies of meiobenthos community in an organic polluted area of Qingdao Bay. J Ocean Univ Qingdao 23: 83–91 (in Chinese) 15. Chen HL, Li B, Hu JB et al (2007) Effects of Spartina alterniflara invasion on benthic nematode communities in the Yangtze Estuary. Mar Ecol Prog Ser 336: 99–110 16. Cai LZ, Li HM, Zou CZ (2000) Species composition and seasonal variation of marine nematodes on Futian mudlat in Shenzhen estuary. Biodiver Sci 8: 385–390 (in Chinese) 17. Guo YQ (2008) The study on the community of free-living marine nematodes in Fenglin mangrove wetlands, Xiamen, China. Acta Oceanol Sin 30: 147–153 (in Chinese) 18. Cai LZ, Li HM, Zou CZ (2000) Species composition and diversity of marine nematode community on intertidal mudflat in Zhongzhai, Xiamen. J Xiamen Univ 39: 385–390 (in Chinese) 19. Zou CZ, Sun GY ( 2002) Studies on the abundance and species distribution of free-living marine nematodes in sand beach, Xiamen Island. Chin J Zool 37: 27–30 (in Chinese) 20. Yeates GW, Bongers T, De Goede RGM et al (1993) Feeding habitats in soil nematode families and genera-an outline for soil ecologists. J Nematol 25: 315–331 8/8







