Supplement K - Genetically Modified Animals & Breeding
advertisement

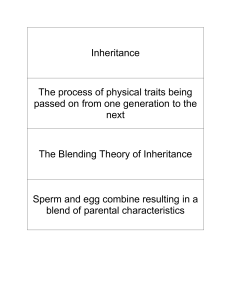
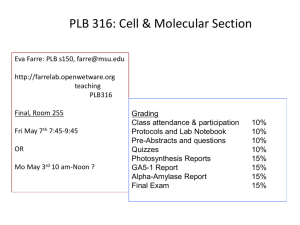
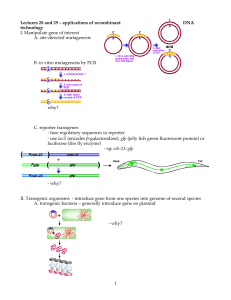
Please enter if known: Protocol #: Supplement K Genetically Modified Animals & Breeding Generation of Transgenic/Gene-targeted Mice* 1. Please list the gene(s) and provide a brief description of its encoded gene products and known activities that will be introduced into the mouse germ line by DNA injection or modified in ES cells by gene targeting. 2. Please describe the expected phenotype. [If unknown, please provide an educated guess based on the known function of the gene(s).] 3. If morbidity develops as a result of the phenotype, please describe steps to be taken to reduce pain and suffering. 4. Please list the procedures to be conducted on the transgenic/gene-targeted animals. Provide detailed descriptions if not described elsewhere in this protocol. unexpected phenotypes that cause disease/distress/pain to the animal develop, you must submit an amendment to the IACUC. Last Revised: 12.29.04 Supplement K-1 Breeding 5. Please list the strains/species that will be bred within the duration of the protocol. 6. Please provide a table/chart that organizes the number expected from breeding. Include all parents and offspring not directly used in experimental procedures. The Division of Laboratory Animal Medicine estimates high for 10 pups per pregnancy. Please use this guideline or give justification for a different estimate. I-1