Soils 206- General Soils
advertisement

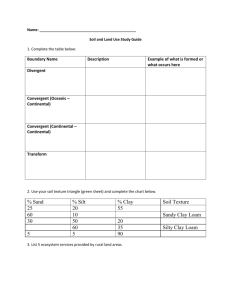
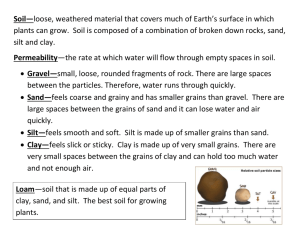
Particle Size Analysis Soils 206- Soil Ecosystem Lab Objectives: 1. Understand the principles and assumptions of Stoke’s Law, 2. Perform particle size analysis using the hydrometer method, 3. Calculate the textural class using the results of the particle size analysis. Soil Texture Soil texture refers to the proportion (by weight) of sand, silt, and clay. The following chart is a generalization of the influences of soil separates on some properties and behavior of soils. Property Diameter Surface Area Inherent Fertility Mineralogy Characteristic, Dry Silt 0.05-0.002 mm Moderate Moderate Often Different Properties of Both Clay <0.002 mm High High Often Different Hard, brittle Properties of Both Plastic and Sticky Water Holding Capacity Drainage Rate Sand 2 – 0.05 mm Low Low Usually Parent Soft, friable (if granular) Non-plastic, Nonsticky Low Fast Moderate Moderate Porosity Compactability Shrink-Swell Potential Sealing Potential Pollutant Leaching Low, Large Pores Low Very Low Poor High Moderate Moderate Moderate Poor Moderate Organic Matter Level Decomposition of SOM Spring Warm-Up Erosion, Wind Erosion, Water Low Rapid Rapid Moderate Low Moderate to High Moderate Moderate High High High Slow (unless cracked) High, Small Pores High High Good Low (unless cracked) High to Moderate Slow Slow Low Moderate Characteristic, Wet Determination of Soil Texture Two methods exist for textural analysis. During a prior lab exercise you practiced using the texture by “feel” or hand-texturing method. Today we will use a mechanical analysis known as the Hydrometer Method. This method involves dispersing a soil sample in water and determining the sedimentation rate of the sand, silt, and clay particles. Sedimentation rates of suspended soil particles depend primarily on particle size. Large sand-sized particles settle faster than smaller clay-sized particles. This relationship can by quantitatively expressed by Spring 2006 1 Stoke’s Law: V = d2 g (Dp – Dl) 18 Where: V = settling velocity (m/s) t = particle settling time (s) L = particle settling distance (m) d = diameter of the particle (m) g = acceleration due to gravity (9.81 N/kg) Dp = density of the particle (2.65x103 kg/m3) Dl = density of the liquid (1.00 x103 kg/m3) = viscosity of the liquid (1.0 x 10-3 Ns/m2) Stoke’s Law can be simplified for our use as gravity remains constant, the particle density and the density of water remain constant and the viscosity of water remains constant. These terms can be combined to form a constant, ‘k’. Entering the known values to the equation and leaving the variable of particle size the equation becomes: (9.81 N kg-1) (2.65x103 kg m-3 – 1.00 x103 kg m-3) * d2 18 (1.0x10-3 Ns m-2) solving, V= V= 9x105 s-1m-1 * d2 or V= kd2 Substituting for velocity and solving for time (t): V= L/ t so t = L/V or t= L/ kd2 Units: t = seconds, L = m, d = m, k = 9x105 s-1m-1 Assumptions of Stoke’s Law: 1. Particles have the same density 2. Particles are spherical, smooth and rigid 3. Particles do not interact with each other or with the walls of the container 4. No Brownian motion 5. No turbulence The time required for a sand particle with a diameter of 0.05 mm or 0.00005 m to fall the distance of 15 cm or 0.15 m is calculated below. t= L/ kd2 t= 0.15 m = 67 seconds 9x105 s-1m-1 * (0.00005 m)2 The time required for a silt particle with a diameter of 0.002 mm or 0.000002 m to fall the distance of 15 cm or 0.15 m is calculated below. t= L/ kd2 t= 0.15 m = 41 780 seconds or 11.6 hours 9x105 s-1m-1 * (0.000002 m)2 Spring 2006 2 Hydrometer method of PSA A hydrometer relies on buoyancy to calculate the number of grams of soil material in 1 liter of suspension at the time of the reading (it has been calibrated to do the mathematics for you). The hydrometer method involves two basic principles; dispersion and sedimentation. Dispersion: Individual soil particles must be separated from each other and remain separated during the determination of particle size distribution. Since aggregates of solid particles are usually held together by some kind of binding agent, it is first necessary to remove these substances, or at least render them ineffective. Once the particles are separated they are said to be dispersed. Dispersion is achieved by chemical and mechanical means. Mechanical stirring is effective in dispersing larger aggregates, but is ineffective on small aggregated clay groups. Chemical dispersion is required in this case. Sodium hexametaphosphate is an effective chemical dispersing agent for two reasons: 1. The sodium monovalent cation replaces polyvalent cations (predominately Ca2+) usually adsorbed on soils thereby breaking one type of interparticle linkage. The polyvalent cations are then reduced in activity by reacting with the phosphorus and precipitating. 2. The adsorbed sodium cations are highly hydrated and raise the electronegativity of colloids until these particles repel each other and remain dispersed. The mixture of dispersed soil particles in water is called a suspension. Sedimentation: Large particles will settle out of suspension more rapidly than small particles because small particles present more specific area and therefore will experience greater frictional resistance. In practice the amount of material still in suspension at any one time is measured with a hydrometer, which indicates the density of the suspension at the hydrometer’s center of buoyancy. Calculations Percent Silt and Clay = Corrected Silt and Clay Average Reading (g/l) * 100 Original Sample Weight Percent Sand = 100% - (Silt & Clay Percent) Percent Clay = Corrected Clay Reading (g/l) * 100 Original Sample Weight Percent Silt = 100% - (Sand Percent + Clay Percent) Use the percent sand, silt and clay to determine the textural class using the textural triangle. Spring 2006 3 Procedure 1. Weigh 50g fine-textured soil (100 g of coarse-textured soil) into a labeled wide-mouth Erlenmeyer flask. The label should indicate the soil name and the precise weight of the soil. Add 100 ml of SHMP (50 g/l), and 50 ml of distilled water. Stir. 2. Quantitatively transfer the contents of the Erlenmeyer flask into a dispersing cup with distilled water. Do not fill the dispersing cup more that ½ full but fill to ½ full, if necessary before mixing. Place on the mixing machines and stir until soil aggregates are broken down. This will usually require 3-4 minutes with coarse textured soils and 5-6 minutes for soils high in clay. 3. Quantitatively transfer stirred mixture to a settling cylinder with distilled water. Fill the settling cylinder to 1000 ml mark with distilled water. 4. Gently mix the suspension using full strokes from the bottom to the top of the cylinders with an agitation plunger. Stir the suspension until the soil material is equally distributed throughout the cylinder, this usually requires 3-4 minutes. Start timing as soon as the plunger is removed from the cylinder. Gradually and carefully insert the hydrometer into the suspension in the settling cylinder. At exactly the calculated time for the silt and clay reading, record the hydrometer reading. Repeat Step 4 three times and record each reading on the data sheet. (NOTE: The hydrometer is calibrated to read in grams of soil material remaining in suspension.) 3. Re-stir the suspension. Take a hydrometer reading at the calculated time for the clay fraction, approximately 11 hours later. 4. Temperature Correction: For each degree above 18 C, add 0.25 to the hydrometer reading. For each degree below 18 C, subtract 0.25 from the hydrometer reading. Note the temperature and the correction for the 40 second readings and for the 11:36 hour reading on your data sheet. 5. Density Correction: Gradually and carefully place the hydrometer you are using in the settling cylinder that contains only the distilled water and the SHMP solution. Note the correction on your data sheet. 6. Following completion of this exercise, the liquid portion can be discarded in sink drains, but transfer settled materials to the soil bucket located in the sink. Don’t forget to leave your labeled soil tin here so it can be oven dried for lab next week! Spring 2006 4