X-ray Diffraction of Cubic structures
advertisement

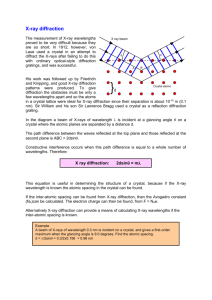
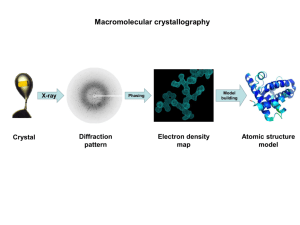
X-ray Diffraction (XRD) Learning Objectives To learn the operation of a x-ray diffraction system To obtain a 2Theta - Intensity (I) scan and index the peaks to identify the crystal structure and its lattice parameters Theory Creation of X-ray’s: When a high-energy electron beam (10-40KV) hits a water-cooled metallic target such as Cu or Mo, the targets emits x-rays corresponding to energies of electronic transitions within the atoms. For example, Cu k (α) = 1.54 A and Mo k (α) = 1.54 A. The x-rays can be made highly monochromatic (very small bandwidth) by using monochromators. What is x-ray diffraction? When the x-rays (E-M wave of low wavelength) impinge on a material, the x-rays interact with the material’s atomic core and the electronic cloud around the atoms. Since the x-ray is an electro-magnetic radiation, it gets scattered by the atoms. The strength of scattering is dependent on the atom itself, i.e., atomic number, Z, and is called the atomic scattering factor. Any solid materials can be classified as single crystal, polycrystalline or amorphous. Depending on the crystalline of the material, the scattered x-rays will interfere, either constructively (crystals) or destructively (amorphous materials). The phenomenon of interference is called diffraction. Diffraction will result only when the wavelength is comparable to or smaller than the distance between the scatterers, i.e., atoms. The diffraction pattern is usually a 3-D pattern and is related to the crystal lattice in a reciprocal way. Actually, it can be shown that the reciprocal lattice (diffraction pattern) is related to a real crystal lattice through Fourier transform, exactly the same way a time domain signal is related to the frequency domain representation. Procedure X-ray diffraction will be conducted on samples (in the Geosciences department) and the data will be given to you in the form of 2Theta vs Intensity (I) scans or data file. You will visit the physics department and learn how it is done. General procedure for indexing and obtaining crystal structures Obtain the 2Theta values corresponding to each peak and tabulate it. Corresponding to each 2Theta value, calculate and tabulate the interplanar distance, dhkl, using Bragg’s law of diffraction: d = λ/ 2Sinθ where: λ is the wavelength of the x-ray. Normalize all the dhkl values to the first one and hence the first one has a unity value. Now compare the normalized (explained in the previous step) value from the experimental data to the normalized data from the structure factor calculation for the various structures (Will be provided by the TA). One set of the structure factor calculation will match with the experimental value. Pick that crystal as structure of the material. Calculate the lattice constant from Bragg’s law for the various hkl’s and average them. Questions 1.) What are the crystal structures (BCC, FCC, HCP, etc…) of Al, Cu, Mg, Zn, KCl, NaCl, and RbCl? 2.) Calculate the lattice constants of these materials? 3.) How would you proceed if the sample is a mixture of materials such as a metal (Al) and a semiconductor (Si)?