Chemical Weathering
advertisement

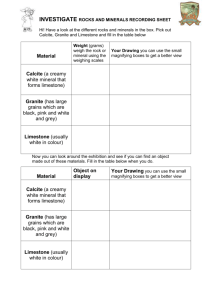
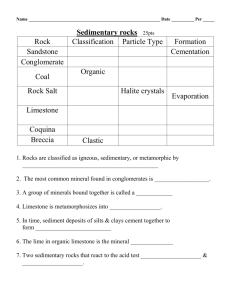
Laboratory Exercise Chemical Weathering One of the most common sedimentary rocks in this area is limestone. Limestone is formed from the skeletons and shells of sea animals, usually microscopic in size. Some limestone deposits are several hundred feet thick. Think how many animals’ remains were deposited at the bottom of an ocean to produce limestone deposits as large as the White Cliffs of Dover in England. These limestone deposits are called chalk. At one time chalk was mined, pressed and shaped, and used in classrooms. Today most chalk is made from a different kind of limestone called gypsum. Limestone under enough heat and pressure form large crystals and gradually change into a metamorphic rock, marble. Limestone is an important raw material of several manufacturing processes. Limestone is used in making cement and smelting iron. Crushed limestone is substituted for gravel and sand in some regions where natural deposits of gravel and sand are scarce. PURPOSE: In this laboratory exercise, you will observe the effects of acids and water on limestone or marble. What effect would this rock as a parent rock material have on the nature of a soil? MATERIALS NEEDED: Limestone or marble chips Hydrochloric acid, 20% Water, distilled Cloth Litmus paper, blue Beakers, 250 ml, 2 Burner Funnel Wire gauze Ring stand Ring clamps Tube clamps Test tubes, 2 Glass tubes, 2, open at both ends (funnel may be used) Rubber bands Graduated cylinder, 100 ml Spoon Evaporating dishes, 4 Medicine dropper PROCEDURE: Work in teams of two as directed by your instructor. Record your observations and answers on the answer sheet provided. Add your sketch to your lab report. A. Place two pea-sized pieces of limestone in an evaporating dish. B. Using a medicine dropper, place four drops of water on a piece of limestone in the dish. Does the water dissolve the limestone? C. Using the medicine dropper, place four drops of hydrochloric acid (20%) on the second piece of limestone. CAUTION: Acid causes skin burns. Use carefully. If bubbles are formed when HCl is added to a rock, the rock is probably carbonate. This is a test for limestone or marble. Observe any change and record the observation. D. At this point you are expected to form a hypothesis about the composition of limestone. This is sometimes called an “educated guess”. A gas is formed when HCl is added to the limestone. What do you think the gas is? Reserve final judgment until more evidence is available. E. Place a piece of cloth over one end of the glass tube. Fasten with rubber bands. Repeat with second glass tube. See sketch Figure 1. F. Fill the two glass tubes with limestone or marble chips that have been washed to remove any dust. Hold upright with tube clamps and a ring stand. G. Place a beaker under each filled glass tube. H. Place 100 ml of distilled water in a beaker. Using a soda straw blow your breath through the water until it will change blue litmus paper to a red color. Pour that water through one of the glass tubes. I. Pour 100 ml of distilled water through the other glass tube. J. Fold a piece of filter paper and place it in a funnel. Hold the funnel in a ring clamp. See Figure 2. K. Place the test tube under the funnel. Pour some of the water solution from one of the beakers through the filter paper. Collect about one-half test tube of the solution. Repeat for the other beaker using clean equipment. L. Take samples from the two test tubes of filtered solution. Take a third sample of distilled water. Place the same amount of water on each of the three evaporating dishes. M. Allow the water from each of the three evaporating dishes to slowly evaporate. Place evaporating dishes on the window sill to allow evaporation over the next few days. Stop back before your next lab and complete procedure N by checking your group’s evaporating dishes. Rapid heating may result in loss of solution. N. Compare the solids left after evaporation. Which had the most material in solution? What was the control in this experiment? What gas absorbed by rainwater helps dissolve limestone? O. When limestone is in solution as calcium bicarbonate, it deposits when the solution reaches a saturation point. Sketch and label a model stalactite and stalagmite. Include this sheet with your lab report.* On the bottom of the page write your explanation of how they are formed from the ceiling and floor caverns. Include the manner in which they grow. Mention the effects on temperature and evaporation. See question 10 QUESTIONS: 1. What gas is formed when HCl is added to limestone? 2. How would water that is slightly acid compare with pure water in the rate of dissolving limestone? 3. How could you tell if your blackboard chalk is made of limestone? 4. What effect does rainwater have as it is absorbed by limestone or limestone soil? 5. What reasons can you give to support your conclusion as to whether there would be caves in limestone? 6. How did reserving final judgment on the composition of limestone in Procedure C affect your final conclusion? 7. Determine how and where the dissolving of limestone has produced changes in topography, cave formation, and other geological formations. 8. What is Karst topography? 9. Name and give an explanation of a famous cavern in the United States. 10. Sketch and give an explanation for Procedure Step O. SUMMARY: Limestone is a very common and useful rock. It is found in large deposits in many places in North America and throughout the world. Limestone is practically insoluble in pure water. If carbon dioxide is added to water, limestone dissolves more readily. Carbon dioxide makes water slightly acidic. The action of water which is slightly acidic, though slow, is responsible for the formation of many caves.