Chemical Reactions Summary
advertisement

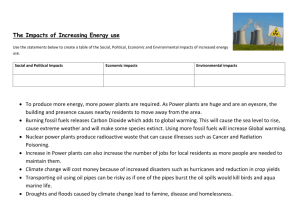
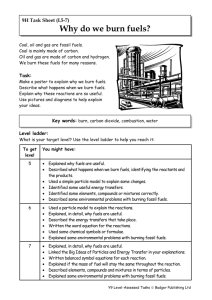
Chemical Reactions Summary Here are the key words from the topic. Make sure that you know what they mean and can spell them. burning oxygen oxide limewater acid burning splint fuel reaction wax oxidation hydrogen smoke equation carbon dioxide water Here are the main facts that you must learn. Look through them and underline the key words. Chemical changes make a new substance and are hard to reverse. They often give out heat. When substances burn, they react with oxygen and make a new substance called an oxide. Substances burn better in pure oxygen than in air because air has less oxygen in it. Another name for burning is combustion. Reactions can be written as equations. e.g. carbon + oxygen carbon dioxide Reacting with oxygen is called oxidation. An oxide contains oxygen. Fuels are substances that burn with oxygen to produce heat. Petrol, wood and natural gas are fuels. Fuels need oxygen and heat to start to burn. When fuels burn, they produce water and carbon dioxide. When metals react with acids, hydrogen gas is made. If a burning splint is put into hydrogen, it explodes with a pop. When carbonates react with acids, carbon dioxide gas is made. Limewater goes cloudy if carbon dioxide is mixed with it. There is oxygen in air. Pure oxygen relights a glowing splint. Now read the key facts again and try this quiz. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. What is another word for burning. If someone put limewater in a test tube, which gas are they testing? What two things do fuels make when they burn? Is melting a chemical change? What two things do fuels need o burn. What gas do metals make when they react with an acid. What is the test for oxygen? What is an oxide? What is the test for hydrogen? Why does a candle go out if a jar is put over it? Name a fuel. Write out the equation that describes the reaction between zinc and oxygen. Read the key facts again now and copy out each of the key words that you underlined.