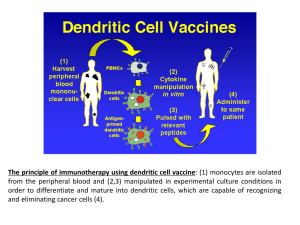
Opdivo - Anthem
advertisement

REVIEW REQUEST FOR Nivolumab (Opdivo®) Provider Data Collection Tool Based on Medical Policy DRUG.00075 Policy Last Review Date: 11/05/2015 Request Date: Initial Request Buy and bill / Policy Effective Date: 11/09/2015 Provider Tool Effective Date: 11/09/2015 / Subsequent Request Individual’s Name: Date of Birth: / / Individual’s Phone Number: Insurance Identification Number: Primary Diagnosis: Diagnosis Code(s) (if known): Ordering Provider Name & Specialty: Individual’s Weight (lbs) (kg) Provider ID Number (if known): Office Address: Contact Name and Office Phone Number: Office Fax Number: Servicing Provider Name & Specialty (If different than Ordering Provider): Provider ID Number (if known): Office Address: Contact Name and Office Phone Number: Office Fax Number: Place of Service: Home Office Dialysis Center Outpatient Hospital Ambulatory Infusion Ambulatory Infusion Center Other: Drug Name/HCPCS Code (if known) Dose to be administered: Nivolumab (OpdivoR) C9453 J9299 (replaces C9453 1/1/16) (mg/kg) J9999 (Other) Other: When did the individual first start this drug? / / Duration: (Weeks) Frequency (Days, Wks, Months) Start Date For This Request: / / This Medical Policy based provider data collection tool is intended to facilitate a UM medical necessity review request for use of nivolumab (Opdivo®) in the treatment of unresectable or metastatic melanoma, metastatic non-small cell lung cancer (NSCLC) and advanced or metastatic (clear cell) renal cell carcinoma. In the appropriate section below, please complete all of the following that apply to the individual. Melanoma □ Request is for the use of nivolumab (Opdivo®) in the treatment of an individual with unresectable or metastatic melanoma (If checked, answer the following as they apply to the individual) □ Opdivo® will be used as a single agent as first-line therapy for untreated melanoma □ Opdivo® will be used in combination with ipilimumab (Yervoy®™) as first-line therapy for untreated melanoma □ Opdivo® will be used as a single agent as second-line therapy □ Opdivo® will be used as a single agent as second-line or subsequent therapy for documented disease progression while receiving or since completing most recent therapy if PD-1 agent has not been previously used □ Opdivo® will be used in combination with ipilimumab (Yervoy®™) as second-line or subsequent therapy for documented disease progression while receiving or since completing most recent therapy if PD-1 agent has not been previously used □ Individual’s current Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 □ Individual has NOT received treatment with another PD-1 agent (for example, pembrolizumab); □ Individual is NOT receiving therapy for an autoimmune disease or chronic condition requiring treatment with an immunosuppressant. REVIEW REQUEST FOR Nivolumab (Opdivo®) Provider Data Collection Tool Based on Medical Policy DRUG.00075 Policy Last Review Date: 11/05/2015 Policy Effective Date: 11/09/2015 Provider Tool Effective Date: 11/09/2015 □ Individual does NOT have human immunodeficiency virus (HIV) infection, hepatitis B virus infection, or hepatitis C virus infection Non-Small Cell Lung Cancer (NSCLC) □ Request is for the use of Opdivo® in the treatment of an individual with metastatic non-small cell lung cancer (NSCLC) (If checked, mark the following that apply) □ Opdivo® will be used as a single agent □ Individual demonstrated disease progression on or after platinum-containing chemotherapy □ Individual has NOT received treatment with another PD-1 agent (for example, pembrolizumab) □ Individual’s current Eastern Cooperative Oncology Group (ECOG) performance status is 0-2 □ Individual is NOT receiving therapy for an autoimmune disease, chronic condition or interstitial lung disease with an immunosuppressant. □ Individual does NOT have human immunodeficiency virus (HIV) infection, hepatitis B virus infection, or hepatitis C virus infection Renal Cell Carcinoma □ Request is for the use of Opdivo® in the treatment of an individual with advanced or metastatic renal cell carcinoma (RCC (If checked, mark the following that apply) □ Opdivo® will be used as a single agent □ Histology confirms RCC with clear-cell component □ Disease progression is demonstrated after 1 or 2 prior anti-angiogenic regimens (for example, axitinib, bevacizumab, pazopanib, sorafenib, sunitinib, etc.) for treatment of advanced or metastatic disease □ Individual has NOT received treatment with another PD-1 agent (for example, pembrolizumab) □ Individual’s current Eastern Cooperative Oncology Group (ECOG) performance status is 0-2 □ Individual is NOT receiving therapy for an autoimmune disease, chronic condition or interstitial lung disease with an immunosuppressant □ Individual does NOT have human immunodeficiency virus (HIV) infection, hepatitis B virus infection, or hepatitis C virus infection □ Other Indications not listed above (Please submit all supporting documents including labs, progress notes, imaging, etc., for review.) This request is being submitted: Pre-Claim Post–Claim. If checked, please attach the claim or indicate the claim number I attest the information provided is true and accurate to the best of my knowledge. I understand that the health plan or its designees may perform a routine audit and request the medical documentation to verify the accuracy of the information reported on this form. / / Name & Title of Provider or Provider Representative Completing Form Date & attestation (Please Print)* *The attestation fields must be completed by a provider or provider representative in order for the tool to be accepted Anthem UM Services, Inc., a separate company, is the licensed utilization review agent that performs utilization management services on behalf of your health benefit plan or the administrator of your health benefit plan. Page 2 of 2