Ionic Compounds
advertisement
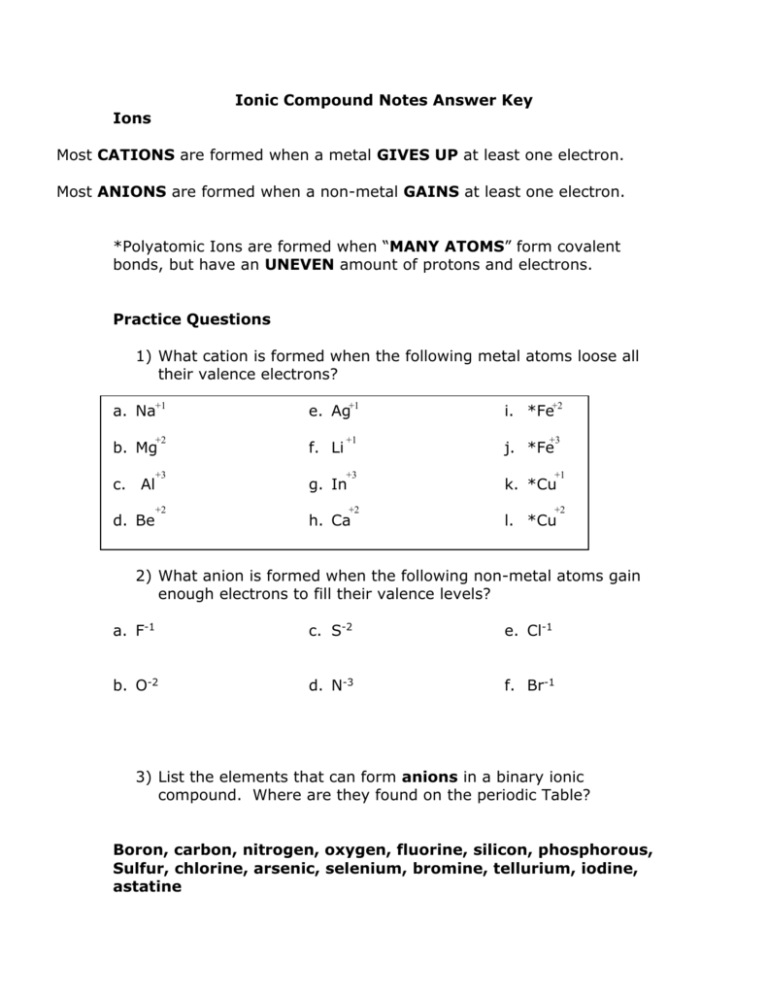
Ionic Compound Notes Answer Key Ions Most CATIONS are formed when a metal GIVES UP at least one electron. Most ANIONS are formed when a non-metal GAINS at least one electron. *Polyatomic Ions are formed when “MANY ATOMS” form covalent bonds, but have an UNEVEN amount of protons and electrons. Practice Questions 1) What cation is formed when the following metal atoms loose all their valence electrons? a. Na+1 e. Ag+1 +2 f. Li c. Al +3 g. In d. Be +2 h. Ca b. Mg i. *Fe+2 +1 j. *Fe +3 +3 k. *Cu +2 l. *Cu +1 +2 2) What anion is formed when the following non-metal atoms gain enough electrons to fill their valence levels? a. F-1 c. S-2 e. Cl-1 b. O-2 d. N-3 f. Br-1 3) List the elements that can form anions in a binary ionic compound. Where are they found on the periodic Table? Boron, carbon, nitrogen, oxygen, fluorine, silicon, phosphorous, Sulfur, chlorine, arsenic, selenium, bromine, tellurium, iodine, astatine Ionic Bonds Ionic bonds are generated because of the ATTRACTION between cations and anions. OPPOSITE charges attract each other because of the coulombic forces. The net charge of all ionic compounds must be ZERO, even though they are made of individually CHARGED particles. For every electron that is missing on the CATION, it must be relocated somewhere else on an ANION. * Polyatomic ions follow this rule, too. Formulas – Practice Questions 4) Write the formula for the compound formed between these cations and anions. a. Mg+2 Br __MgBr2____ e. Ba+2 O-2 __BaO____ b. Ca+2 S-2 __CaS_____ f. Cu+1 O-2 __Cu2O____ c. Al+3 I-1 __AlI3_____ g. Fe+3 Cl-1 __FeCl3____ d. K+1 N-3 __K3N____ h. Ru+3 __Ru2S3__ -1 S-2 5) Write the formula for the compound formed between these cations and anions. These have polyatomic ions in them. Polyatomic are surrounded by parenthesis when there are more than one of the ions in the compound. Ex: Mg(NO3)2 is formed from Mg+2 & NO3-1 ions a. Be+2 IO3-1 _ Be(IO3)2 ____ e. e. Sr+2 NO3-1__Sr(NO3)2___ b. Ag+1 ClO3-1 __ AgClO3 ___ c. Na+1 PO4-3 __Na3PO4___ f. f. Mo+5 SO4-2 __Mo2(SO4)5_ d. NH4+1 I-1 g. g. V+3 OH-1 _V(OH)3_ __NH4I___ Ionic Solids: Crystalline Lattices A CRYSTALLINE LATTICE is formed between the positive and negative ions. Cations are always SMALLER than their original atoms because electrons have been lost. Anions are always LARGER than their original atoms because electrons have been gained. The cations and anions to surround each other in the crystalline lattice. Sodium chloride would look something like this: - + - + + - + - + - Chloride anion (negative) + Sodium cation (positive) - + - + - Different ionic compounds form different shaped crystals because of the ions sizes and arrangements. Practice Questions: 6) Which is a bigger cation: Na+1 or Mg+2 why? #p+ 11 #e- 10 12 Na+1>Mg+2 10 more p+ in Mg Stronger nuclear attraction Smaller ion 7) F-1 or #p+ 9 #e- 10 Which is a bigger anion: O-2 why? 8 O-2 fewer p+ 10 8) Which is a bigger ion: Mg+2 or O-2 why? #p+ 12 8 O-2 fewer p+ #e-10 10 Al+3 or Ga+3 why? e- config Al+3 Ga+3 = [Ar]3d10 Al+3 = [Ne] N-3 or P-3 why? N-3 = [Ne] P-3 = [Ar] Naming Ionic Compounds To go from the formula to the name of an ionic compound: Name the CATION first, then the ANION Include ROMAN NUMERALS for the charge of a metal that has multiple oxidation states. (some transition metals and semi-metals) Binary Ionic Compounds NAME WHAT YOU SEE… ADD “-IDE” Examples: KF = potassium fluoride CuI = copper (I) iodide Cu+1 I-1 Ternary Ionic Compounds NAME WHAT YOU SEE… *if the cation is ammonium, and the anion is elemental, add “-ide” to the anion. NH4Cl = ammonium chloride MgSO4 = magnesium sulfate Practice Naming Given the formula of these binary ionic compounds, determine the proper name. a. MgO c. Na2S e. AuBr b. KCl d. TiN f. Fe2O3 9) Given the formula of these ternary ionic compounds, determine the proper name. a. Mg(NO3)2 b. NH4Cl c. Na2CO3 d. LiC2H3O2 e. Fe(CN)3 f. K2SO4 g. Sn(OH)2 h. Cu2CrO4 To go from the name to the formula of an ionic compound: 1) Determine the CHARGE of the cation. 2) Determine the CHARGE of the anion. 3) Add the right number of cations and anions until the net charge of the compound is ZERO. Use the LOWEST common multiple to determine how many of each ion is needed. 4) Use PARENTHESES to isolate any polyatomic ion if there needs to be more than one of the ions in the compound. magnesium iodide 1) Mg+2 magnesium is an alkaline earth metal, therefore +2 charge 2) I-1 iodine is a halogen, therefore -1 charge 3) add one Mg+2 to two I-1’s to make the net charge 0 MgI2 copper (II) phosphate 1) Cu+2 copper’s charge is +2 because the roman numeral is (II) 2) PO4-3 phosphate is a polyatomic ion – memorize them 3) +2 and -3 the lowest common multiple is 6 so… Cu+2 & Cu+2 & Cu+2 and PO4-3 & PO4-3 Cu3(PO4)2 Practice Formulas 10) Determine the proper formula for these binary ionic compounds. a. Lithium sulfide e. Aluminum oxide b. Sodium chloride f. Iron (II) nitride c. Calcium oxide g. gallium chloride d. Nickel (III) chloride h. tin (II) selenide 11) Determine the proper formula for these ternary ionic compounds a. Lithium iodate e. Cobalt (II) carbonate b. Aluminum nitrate f. Osmium (VIII) hydroxide c. Calcium acetate g. Scandium cyanide d. Molybdenum (V) chromate h. Ammonium phosphide Answers to the practice questions 1)a. Na+1 b. Mg+2 c. Al+3 d. Be+2 e. Ag+1 f. Li+1 g. In+3 h. Ca+2 2)a. F-1 b. O-2 c. S-2 d. N-3 e. Cl-1 3) C, N, O, F, P, S, Cl, As, Se, Br, Te, I, At – they are all non-metals 4) a. MgBr2 b. CaS c. AlI3 d. K3N e. BaO f. Cu2O g. FeCl3 h. Ru2S3 5) a. Be(IO3)2 b. AlClO3 c. Na3PO4 d. NH4I e. Sr(NO3)2 f. Mo2(SO4)5 g. V(OH)3 6) a. magnesium oxide b. potassium chloride c. sodium sulfide d. titanium (III) nitride e. gold (I) bromide f. iron (III) oxide 7) a. magnesium nitrate b. ammonium chloride c. sodium carbonate d. lithium acetate e. iron (III) cyanide f. potassium sulfate g. tin (II) hydroxide h. copper (I) chromate 8) Na+1 has 11p+, Mg+2 has 12 p+, they both have 10 e- - the more protons in the nucleus, the stronger the attraction to the nucleus, the smaller the particle (Na+1 is bigger) Ga+3 has electrons in the 3rd energy level, Al+3 has electrons in the 2nd energy level 9) F-1 < O-2 why? More protons in F, same # e- (10 in each) N-3 < P-3 why? P-3 has e- in the 3rd energy level, N-3 has e-in the 2nd energy level 10) Mg+2 < O-2 why? More protons in Mg+2 (both have 10 e-) 11) a. Li2S b. NaCl c. CaO d. NiCl3 e. Al2O3 f. Fe3N2 g. GaCl3 h. SnSe 12) a. LiIO3 b. Al(NO3)3 c. Ca(C2H3O2)2 d. Mo2(CrO4)5 e. CoCO3 f. Os(OH)8 g. Sc(CN)3 h. (NH4)3P







