Living Cells - Anderson High School
advertisement

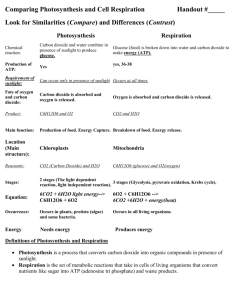
Intermediate 2 Unit 1 Learning Outcomes Booklet 1 - Living Cells Living Cells A unicellular organism is made up of only one cell A multi-cellular organism is made up of many cells An animal cell is made up of Cell Membrane, Nucleus, Cytoplasm A plant cell is made up of Cell Wall, Cell Membrane, Nucleus, Cytoplasm, Vacuole & Chloroplasts The nucleus is the control centre of the cell The cell membrane allows the exit and entry of substances into/out of the cell The cytoplasm is the site of chemical reactions The cell wall is for support and it stops a plant cell from bursting The vacuole holds the plant cell’s sap The chloroplasts are the site of photosynthesis and contain chlorophyll A microbe is a microscopic unicellular organism e.g. a yeast cell Yeast can be used to made bread, wine and beer Alcoholic Fermentation Equation is yeast Glucose ⇨ Alcohol + Carbon Dioxide + Energy Bacteria can be used to make cheese and yoghurt Souring of Milk Equation bacteria Lactose Sugar ⇨ Lactic Acid Antibiotics are produced by Fungi and are used to kill bacteria Diffusion and Osmosis Diffusion is the movement of substances from a high concentration to a low concentration down a concentration gradient. Examples of substances which enter a cell by diffusion are oxygen and glucose Examples of substances which leave a cell by diffusion are carbon dioxide and wastes (e.g.urea) Diffusion is important to cells because - Diffusion provides the raw materials needs for photosynthesis (e.g. carbon dioxide) and respiration (e.g. oxygen) - Diffusion also helps to get rid of waste products Osmosis is the movement of water from a region of high water concentration to a region of lower water concentration across a selectively permeable membrane If you placed a plant cell in a:- Hypertonic Solution - the cell would become flaccid/plasmolysed - Hypotonic Solution - the cell would become turgid - Isotonic Solution - the cell would stay the same If you placed an animal cell in a:- Hypertonic Solution - the cell would become flaccid/plasmolysed - Hypotonic Solution - the cell would eventually burst - Isotonic Solution - the cell would stay the same Plasmolysed is when the cell membrane (also called the plasma membrane) has pulled away from the cell wall because so much water has left the cell by osmosis shrinking the vacuole Turgid is the term used to describe a cell has gained water and feels hard Flaccid is the term used to describe a cell that has lost water and it feels soft and squidgy Respiration The main source of energy in cells is glucose The type of energy stored in glucose is chemical energy Different foods have different energy contents Energy is used by cells for various activities e.g. muscle contraction ATP stores the chemical energy found in glucose An ATP molecule is formed from ADP + Pi The process of glycolysis The process of the breakdown of pyruvic acid Glycolysis produces 2 ATP Breakdown of Pyruvic Acid produces 36 ATP Only 2 ATP are produced in Anaerobic Respiration 38ATP are produced in Aerobic Respiration The end products of aerobic respiration are Carbon Dioxide, Water and Energy The end product of anaerobic respiration in animal cells is Lactic Acid The end products of anaerobic respiration in plant cells is Ethanol and Carbon Dioxide Intermediate 2 Unit 1 Learning Outcomes Booklet 2 Enzymes The 2 products of hydrogen peroxide break down are Oxygen and Water 2 ways that chemical reactions can be speeded up are by increasing the temperature or adding an enzyme 3 properties of catalysts are: 1 – Catalysts speed up a reaction 2 – Catalysts are unchanged by the reaction 3 – Catalysts lower the energy input needed for a chemical reaction Enzymes are Biological Catalysts Enzymes are made in all living cells Enzymes are made from proteins The Substrate is the chemical that the enzyme acts on The Product is the end chemical produced The Active site of an enzyme is where the chemical reaction occurs There is a complementary relationship between the shape of an enzyme molecule and its substrate – the enzyme and the substrate shape fit each other exactly Enzymes are Specific as only one enzyme acts on only one substrate A degradation reaction is a reaction involving the breakdown of a substrate An example of an enzyme involved in a degradation reaction is Catalase Catalase speeds up the following A synthesis reaction is a reaction involving the building up of substrates An example of an enzyme involved in a synthesis reaction is Phosphorylase Phosphorylase speeds up the following Glucose-1-Phosphosphate⇨Starch The substrate of the following enzymes are:- Hydrogen Peroxide ⇨Oxygen + Water Subtrate Enzyme Product Starch Amylase Glucose Hydrogen Peroxide Catalase Oxygen + Water Glucose-1-Phosphate Phosphorylase Starch Low Temperatures slow down an enzyme’s activity High Temperatures slow down and can cease an enzyme’s activity An enzyme is said to be denatured when it’s active site has been damaged pH can increase or decrease enzyme activity Optimum temperature is the temperature that an enzyme works best at Optimum pH is the pH that an enzyme works best at Intermediate 2 Unit 1 Learning Outcomes Booklet 3 - Photosynthesis The type of energy needed for photosynthesis is LIGHT ENERGY from the SUN Light energy is trapped in the chloroplast of a leaf Cholorphyll is a chemical found in a chloroplasts and it traps light energy The energy change that occurs during photosynthesis is Light Energy⇨Chemical Energy The raw materials of photosynthesis are Water and Carbon Dioxide The main product of Photosynthesis is Glucose The by-product of Photosynthesis is Oxygen A summary equation for photosynthesis is:Water + Carbon Dioxide Light Energy Glucose + Oxygen Chlorophyll Diffusion is important to Photosynthesis as it allows carbon dioxide gases in and oxygen out of the leaf cells Photolysis is the first stage of Photosynthesis and it uses light energy from the sun to split water The 3 products of photolysis are Oxygen, ATP and Hydrogen Oxygen is released to the air ATP and Hydrogen are passed onto the second stage of Photosynthesis (Carbon Fixation) Carbon fixation is when carbon dioxide is combined with hydrogen to make the product glucose 2 carbohydrates that glucose can be converted into are Starch and Cellulose Plants need to store Starch so they have an energy store Plants need Cellulose to make the cell wall The factors that limit the rate of photosynthesis are Light Intensity, Carbon Dioxide Concentration and Temperature Horticulturists overcome the problems with limiting factors by:Limiting Factor Method to overcome problem Light Supplementary lighting Carbon Dioxide Use heaters which burn paraffin or propane (they produce carbon dioxide gas) Temperature Add heaters