ETHICS GUIDANCE: PRINCIPLE OF CONSENT
advertisement

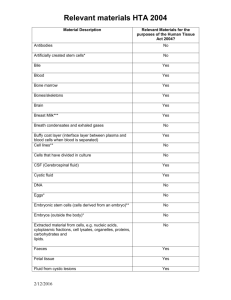
Human Tissue Guidance Factsheet Part 1 Key points (useful links, definitions, how to obtain ethics approval …) What is human tissue? There are many types of human tissue, including skin, body parts, organs, stem cells & bone. Human tissue is defined as: Relevant material that has come from a human body and consists of, or includes, human cells. Relevant materials is defined on the Human Tissue Authority (HTA) website at: www.hta.gov.uk/legislationpoliciesandcodesofpractice/definitionofrelevantmaterial.cfm The HTA has also published a list of what constitutes relevant material: www.shef.ac.uk/content/1/c6/07/21/15/Supplementary_list_of_materials_200811252407.pdf Useful links? Frequently asked questions are at: www.shef.ac.uk/ris/gov_ethics_grp/ethics/practice-.html The homepage of the HTA’s website: www.hta.gov.uk/ For enquiries about the HTA’s work please email: enquiries@hta.gov.uk For enquiries about HTA licenses or license fees or about HTA’s inspections please email: licensing.enquiries@hta.gov.uk The HTA has created a Code of Practice on undertaking research with human tissue: www.hta.gov.uk/legislationpoliciesandcodesofpractice/codesofpractice.cfm Human Tissue Act 2004: www.opsi.gov.uk/acts/acts2004/ukpga_20040030_en_1 The key principle of the Human Tissue Act 2004 is that a person has the right to be asked for consent before any part of their body is used for particular purposes. This applies whether the tissue comes from a living or a deceased person – i.e. consent is the fundamental principle underpinning the lawful removal, use and storage of human tissue. The Human Tissue Act 2004 makes the removing, storing or using of human tissue without consent and the taking and testing of DNA without consent illegal. Please see Annex 4 of this factsheet for answers to the following three questions: - Is consent always legally required to store and use tissue for research? - Should consent be obtained if it is not legally required? - Is consent always required to remove tissue for research? Medical Research Council (MRC)’s Data and Tissues Toolkit: www.dt-toolkit.ac.uk/home.cfm The MRC has published a roadmap to regulatory approval of stem cell research in UK: www.ukstemcellbank.org.uk/documents/InterimUKSCregroutemap120309.pdf Workshop on human tissue, facilitated by the University in the 2007/8 academic year: In 2008 the University’s Research Ethics Committee facilitated a workshop to provide a forum for stimulating discussion of ethical issues surrounding the use of human tissue in research. The report on the workshop, together with presentations that were given, is at: www.shef.ac.uk/researchoffice/gov_ethics_grp/ethics/tissueevent.html At the workshop Professor Sir James Underwood (Emeritus Professor of Pathology & member of the HTA) presented a user-friendly slide which explains a) when consent must be obtained and b) when a license from the HTA is required in order to house human tissue samples. This table can be viewed at: www.shef.ac.uk/content/1/c6/08/21/53/CONSENT%20table.pdf Sheffield Teaching Hospitals NHS Foundation Trust Research Department: www.sth-research.group.shef.ac.uk/research/tissue.html How to obtain human tissue? Human tissue samples can be obtained for research in 3 ways: Last update: December 2009 1 Human Tissue Guidance Factsheet 1. Obtain the human tissue sample(s) from an HTA-licensed bank housing tissue for unspecified research (the research purpose(s) must be specified prior to the use of the tissue) and comply with the HTA-licensed bank’s conditions, which will include the need to: - provide evidence of independent scientific approval; and - comply with the terms of the donor’s consent; and - ensure that the sample(s) are anonymised at the point of release; and - abide by a supply agreement. 2. Submit an application for a specific research project to an NHS Research Ethics Committee (NHS REC). All NHS RECs can ethically review research which plans to use human tissue. The NHS National Research Ethics Service (NRES) governs NHS RECs: www.nres.npsa.nhs.uk/. NHS research ethics applications are submitted via IRAS – www.myresearchproject.org.uk/; The NHS REC will give authority to store the human tissue sample(s) for the duration of the research project. 3. If collecting (i.e. removing) human tissue sample(s) from healthy volunteers (e.g. taking blood from students) then submit an application for a specific research project via the University’s research ethics review procedure. The human tissue sample(s) can only be collected subject to and after informed consent has been obtained from the healthy volunteers and the human tissue sample(s) cannot be stored – the human tissue sample(s) must be processed within 48 hours from the sample(s) having been collected. An NHS REC must ethically review applications for specific research projects: a) if the research will not store human tissue sample(s) in an HTA-licensed bank; and/or b) if the research will store or use human tissue sample(s) without consent. What to do with human tissue at the end of a research project? At the end of a research project there are 3 ways of handling human tissue sample(s): 1. Deposit the human tissue sample(s) in an HTA-licensed bank housing tissue for unspecified research; 2. Apply to an NHS REC in order to use the human tissue sample(s) for a new specific research project; 3. Destroy the human tissue sample(s). The HTA takes the view that disposal of tissue should be the last resort. Obtaining consent to use human tissue sample(s) for secondary research (i.e. for future as yet unspecified research): When applying to an NHS REC in order to undertake a specific research project that will involve collecting (i.e. removing) human tissue from human participants it is good practice to ask the participants’ permission for their human tissue sample(s) to be used for future research (subject to the sample(s) either being stored in an HTA-licensed bank housing tissue for unspecified research or being used on a subsequent specified NHS REC-approved research project). HTA’s position statement on diagnostic archives releasing human tissue for research: www.hta.gov.uk/legislationpoliciesandcodesofpractice/positionstatementondiagnosticarchivesreleasingtissueforresearch.cfm The remainder of this factsheet provides more detailed information on the Human Tissue Act 2004, the HTA and on the HTA’s licensing and inspection processes. If you wish to keep up to date on the work of the HTA then you can subscribe to its e-newsletter (instructions on how to do this are on the HTA’s website). Last update: December 2009 2 Part 2 More Detailed Information The University has the following tissue banks in place, which have been licensed by the Human Tissue Authority (the HTA): Bank housing tissue for anatomy (Designated Individual: Mr David Hinchcliffe) HTA license number: 12130 Bank housing tissue, research purposes to be specified prior to the use of the tissue – located in the School of Medicine (Designated Individual: Dr Simon Heller) HTA license number: 12182 Bank housing tissue, research purposes to be specified prior to the use of the tissue – located in the Kroto Institute (Designated Individual: Professor Sheila MacNeil) HTA license number: 12179 HTA-licensed tissue banks can apply for generic ethics approval: There is no statutory requirement for the ethical review of banks housing tissue for unspecified research. However, HTA-licensed banks housing tissue for unspecified research (research purposes to be specified prior to the use of the tissue) can apply for ethical review on a voluntary basis in order to secure generic ethical approval to conduct research using stored tissue or to release anonymised samples to researchers, subject to certain conditions, without there being a requirement for further applications for ethical review on a project-specific basis. 1 The Human Tissue Act 2004 The Human Tissue Act is a “framework for regulating the storage and use of human organs and tissue from the living and the removal, storage and use of tissue and organs from the deceased, for specified health-related purposes and public display”. The Human Tissue Act applies to England, Wales and Northern Ireland, but the offence of DNA theft applies throughout the UK. The Human Tissue Act’s key provisions are listed at Annex 1. A table that deciphers the Human Tissue Act’s jargon is at Annex 2. The list of scheduled purposes is at Annex 3. Origins and Rationale The existing UK law on retaining and using organs and tissue was reviewed following public inquiries into events at Bristol Royal Infirmary and the Royal Liverpool Children's Hospital (Alder Hey). Together with the Isaacs Report, these inquiries revealed that the storage and use of organs and tissue without proper consent after people had died was commonplace. The legal review showed that the law on tissue retention, both from the living and the deceased, was inadequate and that the law on anatomical examination and transplants needed to be updated. The Human Genetics Commission also raised concerns about the scope for DNA theft. The UK government decided to update the law so that: living patients must consent to the retention and use of their organs and tissue for particular purposes beyond their diagnosis and treatment; there must be consent for the removal, retention and use of tissue from people who have died (given either by those people in life) or in the event that they die without expressing a wish (given by someone nominated by or close to them). 2 The Human Tissue Authority (the HTA) The HTA is the UK government body mandated by the Human Tissue Act to enforce its provisions and the Human Tissue Act authorises the HTA to license human tissue banks. The HTA regulates the removal, storage, use and disposal of human bodies, organs and tissue and issues Codes of Practice and Regulations for the use of human tissue in research. Last update: December 2009 1 The Codes of Practice give practical guidance and lay down the standards expected. The HTA’s Codes of Practice can be viewed and/or downloaded from: www.hta.gov.uk/legislationpoliciesandcodesofpractice/codesofpractice.cfm The HTA licenses organisations that store human tissue for research. One of the HTA’s key aims is to ensure that research continues to thrive in the UK. The HTA believes that good regulation supports good science, which in turn leads to improved healthcare. The HTA hopes that by explaining how we regulate research, we will give people confidence that donated tissue will be put to the best possible use; and this in turn will increase the willingness of the public to donate. By studying human tissue, scientists can improve their understanding of how diseases start and progress, and what keeps us healthy. They may find different ways of diagnosing disease, or develop new treatments. Examples of types of research involving human tissue include: • developing screening tests for different types of cancer; • testing new treatments for conditions like heart disease and diabetes; • looking at how the immune system works to help understand how it combats diseases; • researching how stem cells could be used to treat conditions like Parkinson’s disease and multiple sclerosis. If tissue is removed during the course of treatment or an investigation, there may be some remaining tissue after the procedure that can be used for research. Tissue for research can only be used with the person’s consent. The HTA ensures that this tissue is removed and stored in an appropriate and well managed way. If a person does not want their tissue to be used for any medical research, or they only want it to be used for specific types of research, it is important that they make this clear to the doctor or nurse who asks for their consent, and that they state their wishes in writing on the consent form. A person may also give consent for their tissue to be used for research after their death. If there is no record of the deceased person’s wishes, consent for research can be obtained from someone nominated by them to act on his or her behalf; or, if no one has been nominated, from a person in a ‘qualifying relationship’ – such as a partner, relative or friend. 3 The licensing process The HTA licenses the following activities which involve tissue: i. The carrying out of an anatomical examination; Potential licenses: A storage license; a license to carry out anatomical examinations; a storage license for scheduled purposes. ii. The storage of human bodies for anatomical examination and related research; A license is required for anatomical examination if the human tissue is to be obtained from a deceased person. iii. The carrying out of post mortem examinations, including removal and storage of human tissue; Potential licenses: A storage license for scheduled purposes; a license for the making of a post-mortem; a license for the removal of human tissue. iv. The storage and use of human bodies or parts for public display; Potential licenses: License for public display; a storage license for scheduled purposes. v. The storage of human tissue for other scheduled purposes (e.g. for research). Note regarding tissue used for the scheduled purpose of research: i. A license is not required for tissue that is stored and used for NHS REC-approved specified research projects (the storage and use of tissue for unspecified research requires a license); ii. A license is not required (in respect of transplanting organs) for storing organs for less than 24 hours prior to transplantations Guidance notes and a guide to licensing are available on the HTA’s website. Licensing requirements are set out in section 16 of the Human Tissue Act. The Human Tissue Act requires the HTA to charge reasonable fees for licence applications and to recover costs incurred in ensuring compliance (e.g. the cost of carrying out inspections of human tissue banks). Information on fees is on the HTA’s website. The person completing the HTA license application form needs to: Last update: December 2009 2 i. ii. iii. Specify the activity being covered; (a single license may only cover one activity – see Annex 2 for the definition) Name the designated individual; (see section 4.1) Specify the premises where the activity is to be carried out; (if the premises are at different places additional licenses in respect of each place will be needed) Can a licence cover more than one set of premises? An application for a licence should specify the premises where the licensed activity is to take place. If the licensed activity will take place in premises at more than one place, a separate licence will be needed. Premises in different streets or with different postcodes will be considered as being in different places. In contrast, different buildings on a hospital site could be regarded as in the same place. The HTA will consider this on a case-by-case basis, depending on the facts in each application. When reviewing license applications the HTA will determine the suitability of the activity, the premises and the designated individual. The Compliance Report The HTA’s website contains information on the Compliance Report. The Compliance Report allows people applying for licenses to evaluate the degree to which their human tissue banks meet the HTA’s licensing standards (i.e. the approach to applying for licenses is based on self-assessment). The HTA also uses the Compliance Report as a tool to help decide whether or not an applicant meets the criteria to receive a license. Examples of statutory conditions that are attached to licenses (the ‘givens’): The licensed activity must only take place on premises specified in the licence; The Designated Individual must supervise activities carried on under a licence; The Designated Individual must record information as required; The Designated Individual must keep records as specified; The Designated Individual must provide copies of records/extracts as specified. 4 Key people (Designated Individual; License Holder; Designated Person) 4.1 Designated Individuals A Designated Individual is a person under whose supervision the licensed activity is authorised to be carried on – s/he must be ‘a person who is in a position to ensure that activities are conducted properly, by people who are suitable to carry out those activities, and that all the necessary requirements are complied with’ (e.g. a Head of Department, clinician, scientist, manager, lead anatomist). The person applying for a license must either be the Designated Individual or the Designated Individual must at least consent to it. The Designated Individual must attend one of the HTA’s training courses or complete the HTA’s e-learning package. For further details contact: the HTA: www.hta.gov.uk/ Designated Individuals are named on HTA license applications and are, thereby, authorised by the HTA to supervise activities in licensed human tissue banks. The duties are prescribed in section 18 of the Human Tissue Act, namely to secure that: i. The other people to whom the licence applies are suitable people to participate in the carrying on of the licensed activity; ii. Suitable practices are used in the course of carrying on that activity; iii. The conditions of the licence are complied with. The Designated Individual needs to: Last update: December 2009 3 have knowledge and understanding of the Human Tissue Act and relevant Codes of Practice; have time to undertake the duties; ensure compliance with licence conditions; demonstrate management of the licensed human tissue bank to effect change; have a link to senior management level; know when to seek specialist advice to perform the role. The HTA’s Codes of Practice (e.g. on ‘consent’, ‘removal, storage and disposal of hunan organs and tissue’, ‘import and export of human bodies, body parts and tissue’) are listed on the HTA website at: www.hta.gov.uk/guidance/codes_of_practice.cfm 4.2 License Holder The License Holder: Can be a corporate body; Must have the Designated Individual’s consent to submit the license application; Can apply to the HTA to vary the licence in order to remove the Designated Individual without his/her consent. 4.3 Designated Person Designated Persons are people acting under the direction of a Designated Individual (or person designated by a Designated Individual) and must be named in a notice given by the Designated Individual to the HTA. Other people can work under the direction of Designated Persons. 5 Inspection The HTA will inspect human tissue banks to which it has issued licenses in a proportionate way that is commensurate to potential risk. Inspections will take the form of site visits and desk-based evaluation. During site visits the HTA will gather visual and oral evidence, test the human tissue bank’s compliance against its standards and test the validity of the risk assessment and evidence provided. The HTA has four standards against which it assesses the degree to which human tissue banks are complying with the HTA and Codes of Practice*: Standard 1: Consent Standard 2: Governance and Quality Systems Standard 3: Premises, Facilities and Equipment Standard 4: Disposal The HTA’s Codes of Practice (e.g. on ‘consent’, ‘removal, storage and disposal of hunan organs and tissue’, ‘import and export of human bodies, body parts and tissue’) are listed on the HTA website. Last update: December 2009 4 Annex 1 Key provisions of the Human Tissue Act 2004 i. ii. iii. iv. v. vi. vii. viii. Regulates the removal, storage and use of human tissue; Makes consent the fundamental principle underpinning the lawful retention and use of body parts, organs and tissue from the living or the deceased for specified health-related purposes and public display. It also covers the removal of such material from the deceased, but not from the living; Lists the scheduled purposes for which consent is required (see Annex 3); Establishes a Human Tissue Authority to advise on and oversee compliance; Makes it an offence to have human tissue (which includes hair, nail and gametes in this context) with the intention of its DNA being analysed without the consent of the individual from whom the tissue came, or of those close to them if they have died; Makes it lawful to take minimum steps and least invasive procedures to preserve the organs of a deceased person while consent is sought from next of kin to removing them for transplantation (e.g. procedures such as cold perfusion of organs post mortem are lawful); Gives specified museums in England discretionary power to move human remains out of their collections, if the remains are reasonably believed to be those of a person who died less than 100 years before the date that the relevant provision of the Human Tissue Act comes into force (e.g. allows museums to return human remains to aboriginal groups); Provides a number of exceptions to the general rule that appropriate consent is required in order to store or use human tissue for scheduled purposes. Last update: December 2009 1 Annex 2 Deciphering the jargon contained in the Human Tissue Act 2004 Jargon: Activity Appropriate consent Designated Individual Human tissue (also see relevant material below) Definition: A single license can cover one of the following activities: Anatomical examinations; Post mortem examinations; Removal of post mortem material; Storage of post mortem material for a scheduled purpose; Storage of anatomical specimens; Storage of material from a living person for a specified purpose; Public display. The consent required under the Human Tissue Act – i.e. from the appropriate person as identified in the Human Tissue Act. See section 4.1 of this guidance fact-sheet. What is included? Material that has come from a human body and consists of, or includes, human cells. Examples: - Stem cells from all sources (adult, fetal, peripheral blood, bone marrow, haematopoietic, cord blood and embryonic); - Cellular therapies which use lymphocytes, dendritic cells or other cells. What is excluded? Material from a human body that does not consist of nor include human cells. Examples: - Stem cells that are grown outside the human body and used for research (i.e. transformed stem cell lines); - Hair and nail from living people; - Live gametes and embryos – i.e. because they are already regulated under the Human Fertilisation & Embryology Act 1990. Covers premises that are in the same post code: e.g. the University, Royal Hallamshire Hospital, Western Parkl Hospital, Jessop Wing, and the School of Clinical Dentistry could be considered as one place. Place Premises Buildings. Relevant material See the official definition on the Human Tissue Authority website: www.hta.gov.uk/legislationpoliciesandcodesofpractice/definitionofrelevantmaterial.cfm Scheduled purpose The purposes for which consent is required (with respect to retention, removal and use of human tissue) are referred to as scheduled purposes – see Annex 3. Human tissue used for NHS Research Ethics Committee approved research projects. Specified purpose Unspecified research Research without ethical approval. Last update: December 2009 1 Annex 3 Scheduled Purposes Part 1: Purposes generally requiring consent where tissue is from living or deceased: 1. 2. 3. 4. 5. 6. 7. Anatomical examination (requires witnessed consent in writing before death); Determining the cause of death (except where a post mortem is ordered by a coroner); Establishing after a person’s death the efficacy of any drug or other treatment administered to them (e.g. hospital post mortem); Obtaining scientific or medical information about a living or deceased person which may be relevant to any other person (including a future person) – e.g. genetic information; Public display – requires witnessed consent in writing before death; Research in connection with disorders or the functioning of the human body; Transplantation (includes all bodily material, such as blood, bone marrow, skin, tissue and organs). Part 2: Purposes requiring consent where the tissue is from the deceased: 8. 9. 10. 11. 12. Clinical audit; Education or training relating to human health (includes training in research techniques); Performance assessment (e.g. testing medicinal devices); Public health monitoring; Quality assurance. Last update: December 2009 1 Annex 4 Advice on consent with respect to human tissue: Is consent always legally required to store and use tissue for research? No. There are a number of exceptions under the Human Tissue Act. The most important of these are: Tissue which is an ‘existing holding’ – i.e. it was already held before 1st September 2006; Tissue which has been taken from a living person and the researcher is not able to identify the person and the research is ethically approved by a research ethics committee; Imported tissue. Should consent to use tissue be obtained if it is not legally required? Applicants should consider whether it is ethically appropriate or desirable to seek ethics committee approval. In the case of existing holdings, the HTA’s Code of Practice on Consent includes specific guidance on whether consent should be sought to use of tissue in research (see paragraphs 114-115). Considerations include whether other sources of tissue are available, the feasibility of tracing and contacting donors, the potential for the research to discover information of clinical significance for donors, or to cause them unnecessary further distress. In the case of new tissue from the living, it is good practice to obtain prospective consent to use the tissue in research, where this is practicable. Is consent always required to remove tissue for research? Appropriate consent is always required under the Human Tissue Act 2004 to remove tissue from the deceased for research purposes. Consent is always required under the common law to remove tissue from the living. However, consent from the living may be given for diagnostic or therapeutic purposes. If it is also intended to store and use part of the tissue for research, it is good practice to seek specific consent for research at the same time but this is not a legal requirement. Tissue from the living may be stored and used for research without consent provided the research is ethically approved and the researcher cannot identify the donor(s). Last update: December 2009 1