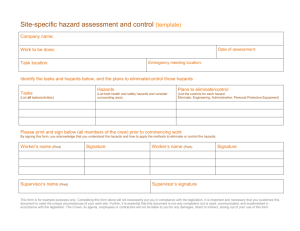
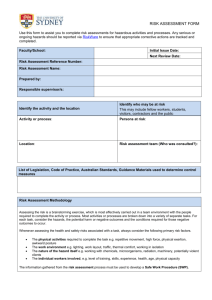
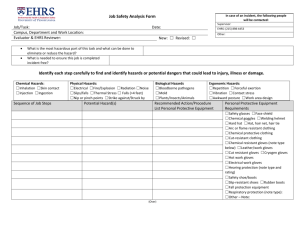
Introduction - University of Nottingham
advertisement

MOLECULAR MEDICAL SCIENCES SCHOOL HANDBOOK To be read and used in conjunction with University Safety Office Guidance and local divisional policies. 1 Please sign a hard-copy of this page and return it to your local safety representative. Name: Position: Department: I have read all the information in the MOL School safety manual and agree to adhere to the policy and guidance within it. Signature: ………………………………………………………………… 2 Index Introduction .................................................................................................................... 5 Statement of Health and Safety Policy ................................................................ 6 Arrangements for Health and Safety and Organisational charts .......... 7-10 Health and safety induction questionnaire ....................................................... 11 Emergency Telephone Numbers and Divisional Contacts .......................... 14 General Office Safety ................................................................................................ 15 Display Screen Equipment (DSE) Safety .......................................................... 15 General laboratory safety .................................................................................. 16-18 Supervision of undergraduate and postgraduate students .................. 16-16 Good Laboratory Practice ................................................................................... 16-18 Experimental practice and design ....................................................................... 19 Cell Culture ................................................................................................................... 20 Centrifuges.................................................................................................................... 20 Compressed Gas Cylinders ..................................................................................... 21 Liquid Nitrogen Vessels ............................................................................................ 21 Safety in Clinical Divisions ...................................................................................... 22 Clinical Trials involving patients or healthy volunteers ............................... 22 Handling of all Blood Products .............................................................................. 22 Venepuncture ............................................................................................................... 22 Work in genetic manipulation facilities .............................................................. 23 Radiation ........................................................................................................................ 26 COSHH ............................................................................................................................ 28 Risk Assessment and hazards ............................................................................... 28 Electrical Safety .......................................................................................................... 29 Guidance for IT staff servicing PC`s in MOL labs.......................................... 30 Fire Regulations .......................................................................................................... 31 Fire Lectures: ............................................................................................................... 31 Queens Medical Centre ............................................................................................ 32 West, South, East & the new ENT Block ........................................................... 32 Medical School ............................................................................................................. 33 Clinical Sciences Building ........................................................................................ 35 First Aid and Serious Injury ................................................................................... 36 Chemical Contamination. ........................................................................................ 36 Accident and Incident Reporting .......................................................................... 36 Manual Handling Operations .................................................................................. 37 School Safety Audit ................................................................................................... 37 Out of hours policy .................................................................................................... 38 Lone working in offices or involving low risk activities………………………..41 New and visiting workers and students ............................................................ 45 Maintenance workers ……………………………………………………………………………47 Children, young persons and adult companions on University premises, assisting with University work……………………………………………………………..47 3 Equipment servicing and decontamination certificate……….………….…48 Immunisation ............................................................................................................... 50 Allergen Screening ..................................................................................................... 51 New and Expectant Mothers .................................................................................. 52 Summary of hazards which may affect the health and safety of new or expectant mothers ..................................................................................................... 55 Risk assessment for new or expectant mothers - see safety office web pages. ............................................................................................................................ .56 Training and competency record for staff and students…………………..56 Competency record for experienced workers...................................................57 Inspection/audit standards……………….……………………………………….…59 4 Safety Policy – Molecular Medical Sciences This manual is intended to compliment the guidance published on the Safety and Radiation Protection office website (http://www.nottingham.ac.uk/safety). The site provides essential university information relating to aspects of Health and Safety on a wide range of subjects. It is recommended that you consult this website in addition to using the MOL Safety Manual and any local polices in your area of work. Introduction. The School of Molecular Medical Sciences comprises of a number of research divisions located at 3 sites, The Queen`s Medical Centre, The Centre for Biomolecular Sciences and the City Hospital. In order to achieve a safe working for all staff and students regardless of their location, the implementation of a single safety document and management system is essential. You are required to read this document and follow the guidance and recommendations within it. The Head of School has appointed a School Safety Officer and Deputy Safety Officer and they are available for consultation and advice relating to policies and procedures to ensure a safe working environment. There are also local safety representatives for various research groups and units. 5 Statement of Health and Safety Policy The School of Molecular Medical sciences recognises the role of those working within it to achieve the highest standards of teaching and research and its role in supporting staff and students to achieve this. An important part of this support is to provide and maintain a safe and healthy work environment for all staff, students and visitors to the School. The Head of School assumes the responsibility for ensuring the effective provision of a safe working environment and in fulfilling this commitment ensures the following: 1. Health and safety will be deemed to be an integral part of school management and function 2. Appropriate resources will be made available to achieve the Schools Health and Safety Policy 3. Effective communication within the school to all staff, students and visitors 4. To include health and safety in the annual school plan 5. Monitor performance to ensure continuous improvement 6. Commit to review and develop the School safety policy 7. Provide adequate and appropriate training as required. The Head of School has appointed nominated staff including the school safety officer, biological safety officer and locally appointed safety representatives, to assist in fulfilling his responsibilities. The implementation of the policy within specific areas is the responsibility of Academic staff and Principal investigators in line with their managerial duties. All staff and students are required to assume responsibility for their own safety and for that of all other personnel that may be affected by their work activities. Work carried out within the school and in certain activities within other parts of the University must be risk assessed prior to work commencing and appropriate documentation completed in accordance with school and university policy and any significant risks addressed by procedural change or effective safety measures. Personnel are required to maintain good levels of housekeeping within all areas and unsafe practices will be dealt with as appropriate. This statement of intent is supported by the contents of this document and other University polices of which all personnel must read and implement. All staff, students and visitors must be fully aware of all procedures and polices that relate to their work. Signed Head of School Date February 2010 Date to be reviewed January 2011 6 Arrangements for Health and Safety Head of School and Management Group. The Head of School and MOL Management Group has overall responsibility for Health and Safety for all personnel within the school. The main duties are: ●To receive and act upon the minutes received from the School Safety Committee ●To receive a quarterly report from the school safety officer ●To communicate any recommendations to the appropriate personnel ●To ensure provision of appropriate and sufficient resources for the overall maintenance and development of health and safety. MOL Safety Committee. The role of the committee is to oversee the day to day management and provisions for health and safety throughout the school. The main duties are: ●To report to the Head of School and Management Group regarding the management of health and safety in MOL ●Review safety polices, arrangements and documentation on a regular basis ●Outline appropriate proposals for improving and maintaining safety performance ●Review audits and inspections to determine that agreed standards are being met ●Review accident and near miss reports and make recommendations on remedial action ●Ensure that current legislation and polices are complied with and included in relevant school documents ●Consider any new research proposals that may have any implications for health and safety including space requirements and the purchase of new equipment School Safety Officer The School Safety Officer is responsible for the coordination of Health and Safety within MOL, reporting to the Head of School and the School Management Committee. The SSO chairs the MOL Safety Committee and attends the Faculty of Medicine Safety Committee and the Trust Pathology Health and Safety Group. Divisional Representatives The Laboratory Safety Representatives have responsibility for the day to day monitoring and regular inspection of their laboratory areas to ensure that they are operating in a safe manner. Any concerns can be raised with the School Safety Officer who will advise and assist with appropriate advice and actions. 7 Radiation Protection Supervisors All RPS are appointed for areas where radioactive materials are handled. They are responsible for ensuring the safe management of all radioactive sources within their areas. No work may start without the written authorisation of the Safety Office and the registration of both the project and personnel carrying out the work. Raising Health and Safety Issues If you have any concerns or issues with any aspect of Health and Safety within MOL or in any area in which you are required to work, you should raise them in the first instance with your immediate line manager. If further assistance is required then contact the SSO : Julie Stanley on 31858 or email Julie.stanley@nottingham.ac.uk or the appropriate representative on the MOL Safety Committee. The University Safety Office is always available for advice on 13401 or by email safety-office-enquires@nottingham.ac.uk A list of divisional contacts can be found on page 14. Induction Training and competency testing All new members of staff, students and working visitors are required to undertake appropriate induction safety training as outlined in the MOL Safety Manual. All inductions must be completed prior to the commencement of any laboratory work and recorded. In addition most individual research groups will carry out training relevant to the procedures and operation of equipment in a particular area. Forms to be used to record training and/or competency tests for new or experienced research staff can be found at the end of this document and are also available on the MOL web site. It is essential that training must be accompanied by a competence test for new staff which must be documented on the training record. Forms must only be signed by the person delivering the training and assessing competency. 8 9 10 Induction All new staff, students and visiting workers must undergo an induction in their work area and complete the induction form below. A signed copy must be kept with Divisional Safety Officers and/ or Academic Supervisors. In addition a safety induction checklist is also available on the MOL safety web page and includes items that must be covered as part of this process, with the ability to include specific items relevant to the area. Molecular Medical Sciences Health and safety induction questionnaire Name......... ......... ......... ......... Position/student........ ......... ......... ......... Date first employed......... ......... ......... Laboratory location……………………………….. This questionnaire is designed to ensure that you have understood all the basic safety rules and procedures in the area in which you work. You may be required to undertake more than one induction. You should be able to complete all sections. I have attended a safety induction session on………………………… I have received the school safety handbook and understood the contents I am familiar with the local fire evacuation and other emergency procedures I attended a QMC fire talk( for embedded units ) session on................................. A. Fire A1. What action should you take if you discover a fire? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... A2. What action should you take on hearing an intermittent high-pitched alarm? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... A3. What action should you take on hearing a constant high-pitched alarm ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... A4. Where is your nearest means of raising the fire alarm? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... A5. When should you evacuate QMC/City or the CBS building ( delete as applicable) 11 ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... A6. What is your fire escape route and assembly point? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... A7. Why should fire doors be kept closed? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... B. Accidents / First Aid B1. Where is your nearest first aid box? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... B2. Who is your first-aider and how would you contact them? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... B3. What is the procedure for reporting accidents in your area? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... B4. What is the internal emergency telephone number from your area? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... B5. What information would you give if you telephoned the above number? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... C. Safety Responsibility / Procedures C1. Who is your divisional Safety Officer and how would you contact them? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... C2. Who are the divisional Safety Reps and how would you contact them? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... C4. Where would you find out more information about health and safety issues? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... D. Your work and safety D1. The table below gives 15 hazards which are common in the School. Tick those which you might encounter in your line of work? Biological hazards..... Clinical waste ..... Lasers ..... Chemical hazards..... Chemical waste ..... Noise ..... Radiation ..... Lab equipment..... Waste Disposal ..... 12 Flammables ..... Display screen equipment ..... Manual handling ..... Sharps ..... Electrical equipment ..... Work at height ..... D2. State any other hazards specific to your work? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... D3. Describe any health surveillance which your department requires you to undergo? State the frequency and where the surveillance is carried out? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... D4. What type of personal protective equipment are you required to use for your work? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... D5. Describe the procedure for reporting of hazards or faults which you discover at work? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... E. Miscellaneous E1. Give reasons for maintaining a clean and unobstructed workplace? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... E2. What checks should you make before using any work equipment? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... E4. If you bring in electrical equipment from outside, what action should you take before using it? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... E5. Will your work involve much lifting and carrying? If YES, have you attended or intend to attend the handling and lifting training course? ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... ......... E6. List the disposal routes for waste that may be generated by your lab. a. Decontaminated plastics…………………………………………………. b. Broken glass…………………………………………………………………….. c. Clinical waste/blood…………………………………………………………. Signed Dated Name of supervisor 13 Emergency Telephone Numbers and Divisional Contacts QMC CHN Adult A&E 43671,-75,-76 43681 Eye Accident and Emergency 42882 42882 Adult Cardiac Arrest 2222 2222 Paediatric Cardiac Arrest 2222 2222 Security 43335 49999 Fire Officer 42399 46899 Fire Alarm 2222 2222 University Safety Office 13401 13401 External Emergency Services 9-999 9-999 University Radiation Protection Officer 13402 13402 Health and Safety Executive 9-9470712 9-9470712 Occupational Health 44342 46657 Cripps Health Centre 13475 13475 Julie Stanley - Oncology 31858 E mail Julie.stanley Patrick McClure - Microbiology 30750 Patrick.mcclure Neil Oldfield – Microbiology (CBS ) 30742 Neil.oldfield Sue Bainbridge - Immunology 30726 Sue.bainbridge Darryl Jackson - Pathology 30080 Darryl.jackson Di Mitchell – School Manager 67590 Diane.mitchell Tamar.GuettaBaranes Tamar Guetta-Baranes, Clinical Chemistry Andy Green - Pathology 30786 14 Andrew.green General Office Safety In offices, trips and falls are a common source of accidents. Therefore, you should keep all floor areas free from goods, litter and trailing cables. Step stools or ladders must be made available for access to high level storage. If you require the separate administration manual please contact Julie Stanley or Di Mitchell. Care must be taken with all office equipment e.g. fax machines, photocopiers and guillotines to avoid unnecessary accidents. Follow instructions given in the equipment operating manuals. Display Screen Equipment (DSE) Safety The School implements the University policy concerning DSE in order to provide a safe environment for staff whose work is highly dependent on the use of DSE. Guidelines are updated on the University Safety Web pages. The following guide will help you decide if you are a DSE “user” under the current guidelines. The Guidelines used by the University are: User Anyone whose work involves using a computer more than 10 hours per NonUser week. Anyone whose work involves using a computer between 5 and 10 hours a week where sessions can last 90+ minutes or where the work is very intensive. Anyone whose work involves using a computer less than 5 hours a week. Anyone whose work involves using a computer between 5 and 10 hours a week where the sessions last less than 90 minutes or the work is not intensive. If you are a DSE User, what should you do now? Go to the University Safety Office website as soon as possible and read the Guidance at: http://www.nottingham.ac.uk/safety/dse-safe-use.htm Then, either complete the on-line training available (suitable for most Users) from the Safety Office Guidance or attend the training course in Safe Use of DSE provided by the Safety Office (recommended for intensive Users). If you have previously had DSE training, you need only do this if you feel that you need a refresher course. Complete the Self-assessment Workstation Checklist which can be completed on line and submitted to Sue Bainbridge, Immunology, The URL is: http://www.nottingham.ac.uk/safety/local/dsechecklist.htm If the need for further action is identified, a meeting will take place to agree the best course of action. 15 General laboratory safety Tidy working conditions are essential to safe working practice. Keep equipment/materials not in use out of harms way - in drawers or cupboards if possible. Keep only the minimum amount of paperwork in laboratories and leave equipment and work surfaces clean and tidy. Keep trailing wires or tubing as short as possible and tidy. Laboratories are considered restricted areas and only suitably qualified personnel working to Good laboratory Practice should enter them. The only exception to this is where patients or normal volunteers are being studied and when external service engineers are on site. Staff should ensure that these people are supervised at all times when it is necessary for them to enter laboratories. The main hazard to health in clinical divisions is a risk of infection from procedures involving patients and healthy volunteers as well as from the handling of biological samples. It is therefore ESSENTIAL that anyone with broken areas of skin who is in contact with the laboratories always keeps these areas covered with either waterproof plasters or disposable gloves. Use automatic pipetting devices at all times. Supervision of undergraduate and postgraduate students It is the responsibility of all student supervisors to ensure that the student is fully aware of all safety issues relating to their research and that the project assessment form is completed and an induction and training record maintained. All required forms are available from the MOL web safety pages. A document containing the safety responsibilities expected of principle investigators and senior managers is also available on the MOL safety pages. For all projects that involve handling of human or primate blood immunisation against Hepatitis B is recommended and can be organised through Occupational Health. Good Laboratory Practice Laboratory coats Laboratory coats MUST be worn and fastened at all times whilst in a laboratory and must not be worn in any area other than a designated laboratory or store, and certainly not in general circulation or eating and refreshment areas. The Safety Office recommends the wearing of Howie Style lab coats that have a cuffed sleeve and a high fastening neck for work involving chemicals of a hazardous nature. For further guidance see the link below on the safety office site: http://www.nottingham.ac.uk/safety/guides.htm#labcoats All lab coats not in use must be left within the designated laboratory or corridor. Never enter an office wearing a lab coat or place one on the back of a chair Laboratory coats must be laundered at regular, frequent intervals. Wear appropriate 'normal' clothing under your laboratory coat when in the laboratory. While a 'normal' pattern of laboratory coat offers some level of protection to the individual, it cannot be classed as personal protective equipment (PPE). It will not protect legs or the neck 16 area, and at best will only prevent your clothes from becoming lightly contaminated. You need to take this into account when designing your experiment. It is not recommended that shorts or short skirts be worn in chemical laboratories. Appropriate footwear must be worn at all times in the laboratory. This is defined as shoes or boots that enclose the whole foot. Sandals or other open-toed shoes are not appropriate laboratory footwear. When involved in handling heavy loads, safety footwear must be worn. Anyone involved in manual handling must attend the Safe Handling Course run by the Safety Office. Eye Safety Safety glasses or goggles must be worn whilst in a laboratory, where risk assessment for any process on-going indicates that they should be worn (eg. carrying out a chemical reaction) Certain routine operations may not call for such eye protection, but you need to check what others in the immediate area are doing, and adopt any safety measures as their work may call for. Normal prescription glasses are NOT a suitable substitute for safety eye-ware. Prescription safety glasses are available and your School can help with the cost of these items. You are strongly advised not to wear contact lenses in the laboratory as these can delay effective first aid. Gloves Biological Hazards: Gloves are provided as a barrier from biological hazards. Common types of gloves suitable for work with biological agents are stocked in the Medical School Stores. In depth information to help you select the correct glove for your application can be found on the University Safety web site. Some individuals may suffer sensitivity or dermatitis from different materials and should experiment with all available to determine which is best for them. Chemical hazards: gloves appropriate to the task performed should be worn and suitability should be addressed in your risk assessment. Adequate protection must always be worn when handling hot objects or cryogenic materials. Be aware of the different types of glove suitable for protecting from hot and cold hazards. Guidance is available from the University Safety website to help you with your choice of glove: http://www.nottingham.ac.uk/safety/docs/Gloves_Selection.doc . In some laboratory settings, personnel need to move between laboratories when carrying out certain methods, often within rooms frequented by non-laboratory workers (e.g. Lecturers, cleaning staff etc). In this instance the individual should remove a glove from the hand they intend to use to open the door to avoid potential spread of biological and chemical hazards. It is recommended that this rule should be followed even with clean gloves to avoid any confusion, particularly with new students unfamiliar with contamination control practices. 17 Food, drink and ingestion Food and drink of any variety must not be taken into any laboratory. Eating and drinking utensils should only be kept in designated coffee rooms and offices. Cosmetics or topical medical preparations must not be applied in a laboratory. Never inhale, taste, or swallow any chemical and never pipette liquids by mouth; always use a mechanical filler. Be careful during the fitting process. Use of mobile phones and I pods. The use of mobile phones and I pods must be restricted to an office environment. It is not appropriate to use them in a laboratory area for the following reasons: There is a high risk of them becoming contaminated with any of the hazardous substances being handled in the laboratory. This may then result in exposure of the individual via ingestion or skin contact. Contamination can also be carried out of the laboratory thus putting others at risk. They are cause of distraction, which is a potential hazard when carrying out safety critical procedures Where it is important to be aware of what is going on in the work area. Wearing ear phones interferes with the ability to hear alarms and to conduct normal communication. Labelling of materials in use All containers must be clearly labelled with their contents and appropriate safety warnings, date, name and supervisor identified. This label must be durable and must be replaced if showing signs of deterioration. Felt-tip and other spirit markers are not acceptable for labelling glassware directly. For safety reasons, unlabelled containers are likely to be disposed of without reference to any user. Make sure that all of your personal commercial chemicals and other materials are coded with initials relevant to you, your supervisor or your research group, and the month and year of purchase. Transportation of chemicals Chemicals should be transported in appropriate secondary containers, for example, Winchester carriers or sample trays or stacker boxes. 18 Trolleys or solvent chests are to be used for large, heavy or multiple item transport. Please ensure that the correct trolley is selected e.g. cylinder trolley, a trolley with bottle containment or a sack truck. Chemicals and hazardous materials are to be transported between floors of a building using the goods lift and not the staircases. See page 17 for instructions on moving liquid nitrogen Glass and sharps Glassware must always be handled carefully and inspected before use. Worn or damaged glassware is particularly liable to break and should be disposed of. Glass pipettes fracture easily and great care should be taken when fitting them into safety pipette fillers or dispensers. Any glass container presents a physical hazard in its own right, particularly if it becomes broken. For this reason, the secondary containment of all glass contained materials prior to transportation is crucial. The main causes for cuts can be eliminated by taking account of the following: Do not leave glass or metal sharps lying around. Non- contaminated and contaminated syringe needles and sharps must be placed in the sharps containers provided. When 3/4 full these containers should be sealed safely, and disposed of via local arrangements. You must use the carriers provided when transporting Winchesters. The stores have instructions not to supply Winchesters unless the customer has an approved carrier. Always soften plastic tubing with hot air or hot water before attaching to glass tubing; lubricate the plastic or rubber tubing with grease or water and protect your hands with heavy gloves or a heavy cloth while inserting the glass tubing and avoid using excessive force. Remove plastic or rubber tubing from glass by cutting it off and use adequate personal protection during experimental work. Experimental practice and design All experimental work must be discussed with commences. An assessment of all risks, including must be performed prior to commencement of a procedures must be checked to make sure that modifying an existing one. 19 an academic or supervisor before work chemical, physical and mechanical hazards novel procedure. Assessments for existing they are still valid - especially if you are The Control of Substances Hazardous to Health (COSHH) regulations requires a written protocol to be prepared for every experimental procedure before commencement. Make sure that you are aware of what materials fall under this set of Regulations. It is the responsibility of each user to ensure that their bench area and laboratory are kept clean, tidy and in a safe condition. Cell Culture In addition to GLP, workers using human or animal cells or primary cultures MUST work within these safety guidelines. It also a requirement that the guidelines laid down by the Safety Office are adhered to. Work in the Cell Culture laboratories should not commence unless the worker knows what he/she is doing and has informed the Safety Officer of any specific safety procedures. When performing cell culture, workers must use the correct Howie-style laboratory coat which has a high neck and cuffed sleeves. As far as is practical, all manoeuvres involving the handling of human tissue/cells must occur in the Class II hoods. Transport of cultures to incubators should be in closed containers to avoid spillage. All centrifugation should be performed preferably in sealed rotor buckets and where possible these should be loaded and unloaded in the Class II hood. Each worker has sole responsibility for the safe disposal of sharps and both solid and liquid waste and for the decontamination of glassware and apparatus generated by his/her work. Thermometers Non mercury thermometers are available from the medical school stores and it is preferable to use these instead of the older mercury filled ones. In the event of a breakage of a mercury thermometer, the spilled mercury must be contained at once using a mercury spill kit. These kits are available from pharmacy at both City and QMC sites and can be returned to pharmacy for disposal. Centrifuges Workers should never attempt to use a centrifuge unless they have been previously instructed in its safe operation and that training is recorded on the appropriate training record. Centrifuge buckets, adapters, bowls and windshields should routinely be wiped or washed out with a mild disinfectant. 20 Any contamination should be dealt with as soon as possible by wiping or soaking all contaminated parts in mild disinfectant. Should a breakage occur during centrifugation, the centrifuge should be left unopened for at least 30 minutes to allow any aerosols formed to be dispersed and then be thoroughly cleaned with mild disinfectant. All body fluid samples must be spun in sealed rotors at all times. Balance the load on the centrifuge accurately before switching on to avoid putting undue stress on the bearings and keep the centrifuge clean and dry. This includes the interior of rotors as corrosion can seriously weaken the equipment. Where such apparatus is exposed to corrosive atmospheres frequent and stringent inspections should be made. Compressed Gas Cylinders Gas cylinders are present in most laboratories and it is essential that they are adequately secured against falling (either in a stand or clamped to a bench). The correct regulator must be fitted and the door to the area containing the cylinders must display a suitable warning notice. Regulator valves must be inspected regularly and either serviced or replaced on a five yearly basis. A record of all regulators should be kept on the departmental equipment inventory, recording date of purchase and when they are either serviced or replaced. A new standardised colour scheme was introduced in Autumn 2005 and details are available on the Safety Office web pages. They must only be handled and the regulators changed by personnel who have attended the University Cylinder Handling Course. Regulators must be inspected on a regular basis and appropriate records kept. All regulators must be replaced after a period of 5 years or sent for inspection off site to confirm they are still safe to use. More detailed information is available on the University safety web site. http://www.nottingham.ac.uk/safety/publications/circulars/index.html#Compressedgases Liquid Nitrogen Vessels You must protect your hands at face at all times when in contact with liquid nitrogen. Liquid nitrogen is extremely cold (boiling point -196oC) and extreme care must be taken when handling, storing, transferring liquid nitrogen, or any objects which have come in contact with it Proper safety attire must be worn i.e. face shield, cryogenic gloves and apron. Handle any equipment that has been exposed to liquid nitrogen with tongs. Immediately remove any clothing on which liquid nitrogen is spilled or splashed medical attention should be sought for any frostbite injuries that may occur. Extreme care should be taken when inserting or removing samples from the liquid nitrogen vessels. Both these procedures should be done slowly. When removing samples, allow any liquid nitrogen to run off before lifting clear of the vessel. Vials and boxes designed for cryogenic use must be used at all times and in no circumstances try to substitute other plastic items. Take extreme care when transporting liquid nitrogen and make sure that only Dewar type containers should be used. Ensure that the container is thoroughly dry before adding liquid nitrogen and never overfill. Only use the goods lift when transporting liquid nitrogen between floors. Personnel must not ride the lift with a loaded liquid nitrogen dewar and must ensure a policy is in place to send the dewar and lift between floors whilst prohibiting staff from entering 21 the lift. Never use liquid nitrogen in confined spaces and always make sure there is adequate ventilation. When obtaining liquid nitrogen from the Medical School Stores it is advised that two members of staff attend, in case there is a major spillage. Doors to the loading bay must always be left open when dispensing from the large dewar. Members of staff should be encouraged to go on the University "Safe handling of cryogenic liquids" course. UNDER NO CIRUMSTANCES IS LIQUID NITROGEN TO BE HANDLED OUT OF HOURS WITHOUT A BUDDY SYSTEM IN PLACE Safety in Clinical Divisions The main hazard to health in clinical divisions is a risk of infection from procedures involving patients and healthy volunteers as well as from the handling of biological samples. It is therefore essential that anyone with broken areas of skin who is in contact with the clinical areas always keeps these areas covered with either waterproof plasters or disposable gloves. A wide variety of powder free gloves are available depending on the procedure carried out, an individual’s skin sensitivity or personal preference. Clinical Trials involving patients or healthy volunteers No clinical trial work may start without appropriate committee approval, and written consent from the subject or volunteer. When drugs are to be administered to subjects outside the normal indications, and where there is the possibility of an adverse reaction, two members of staff should be present, one of whom should be medically qualified. Divisions carrying out invasive procedures and/or giving drugs should have appropriate resuscitation equipment nearby and a system should be in place to ensure that the equipment is regularly checked. Handling of all Blood Products All tissue and body fluid samples from human and animal sources must be treated as being potentially hazardous. Any spillages should be wiped up immediately with 2 % Trigene. Venepuncture Only trained personnel trained in the technique of venepuncture and the dangers associated with it are permitted to take blood. Unless essential for the research being undertaken, blood should NOT be taken from normal volunteers who have had serum hepatitis, any form of jaundice, recent glandular fever (within the last three months), a history of fainting or any 22 unexplained fever. Blood should only be taken in a designated area preferably with a washable floor. All donations must be recorded and a form can be acquired from safety officers within the school. There are guidelines for the amount of blood that can be taken per donation, 50mls, and 500mls total over a period of 6 months. Work in genetic manipulation facilities Registration of work Discuss the nature of the hazard with the School Biological Safety Officer, and consult the University guidance published on the Safety Office web-site. All work must be assessed by the principal investigator and submitted to the NI of the appropriate GMSC. A list is available on the Safety Office web site. No work may commence without approval. All supervisors must be registered to work on the specific project, and must be satisfied that the project has been assessed, approved and registered by the committee and that all the workers have been registered. It is the supervisor`s duty to ensure that the laboratory facilities available meet the requirements for the required level of containment identified in the assessment and to ensure that training in good microbiological practice has been received by all workers involved. They must be aware of and take precautions against any hazard involved and these must be clearly communicated and discussed. Individuals with compromised immune systems, known allergies or undergoing antibiotic treatment may be at increased risk form hazards involved in these types of project. The supervisor must ensure that any laboratory practice, including disinfection and disposal, meet the requirements of the local rules and is responsible for reporting all incidents and accidents. Where the work is at containment level 2 or above, the supervisor must maintain a list of cultures in use and in storage. Recommended laboratory practice for work at Containment Level 1 The following laboratory practice should be followed for areas used for containment level 1 work: Laboratory personnel must receive instruction in the procedures conducted in the laboratory. A responsible member of the laboratory staff must supervise the work of all new entrants. The laboratory should be easy to clean and bench surfaces should be impervious to water and resistant to acids, alkalis, solvents and disinfectants. 23 The laboratory must contain a hand-wash basin or sink that can be used for hand washing only The laboratory door must be closed when work is in progress and when the lab is vacant, the door should be kept locked. Eye-protection must be worn at all times when working in the laboratory with disposable gloves worn and changed regularly. Discard the gloves for disinfection or autoclaving after use, and not in the ordinary black bag waste bins. Suitable laboratory coats such as the Howie-style must be worn in the laboratory and must not be removed from the laboratory. Mouth pipetting must not take place, use a mechanical filler at all times. Hands must be washed with a disinfectant hand-wash immediately when contamination is suspected, after handling viable cells and before leaving the laboratory. Procedures must be performed to minimise the generation of aerosols and effective disinfectants must be immediately available in the event of a spillage. Bench tops must be left clean, tidy and safe after use each day. Used laboratory glassware and other materials awaiting disinfection must be stored in a safe manner. Pipettes and pipette tips if placed in disinfectant must be totally immersed. All waste material must be made safe by a suitable procedure before disposal and materials for disposal must be transported without spillage in robust containers. All accidents must be reported via the supervisor or your divisional safety officer. Doors of laboratories in which genetic manipulation is carried out must be labelled "Biohazard" and "Authorised Personnel Only", and must be closed shut when work is in progress. Recommended laboratory practice for work at Containment Level 2 As for Containment Level 1, but in addition: Access to the laboratory is limited to personnel and other authorised persons. If the laboratory is mechanically ventilated, it must be at negative pressure relative to other areas onto which it opens - the exhaust air from the room must be ventilated to the atmosphere safely. An autoclave for the sterilisation of waste materials must be readily available. Recommended laboratory practice for work at Containment Level 3 There are specific requirements for containment level 3 work and advice should be sought from MOL academics and researchers in conjunction with the University Biological Safety Officer. Disinfection, Sterilization and Disposal of Microbiological and Cell-culture Waste. Rules for dealing with solid and liquid biological waste, mixed chemical and biological waste, contaminated instruments and equipment, spills of biological samples and available 24 disinfectants are arranged locally. 25 Radiation General All work involving the use of radioactivity is regulated by the Ionising Radiations Regulations 1999 (IRR99). The University has detailed radiation safety rules which are published on the Safety Office web-site. The Safety Office must be pre-notified of any intention to commence work with radiation sources for the first time, or at a new location, or with new sources, or for a new application using existing sources. Each division working with radiation sources must appoint at least one Radiation Protection Supervisor who will develop a set of written Local Rules for safe working with radiation. Divisions must register radiation workers with the Safety Office using the University Registration Form available on the University Safety Web site. Each division must pre-notify to the Safety Office of the intention to work with radiochemicals and prior to working with radiation sources, a risk assessment must be completed and written procedures for use produced. No radiation work may be carried out without the knowledge and consent of the Radiation Protection Supervisor and the Safety Office and all radioactive work must only be carried out in supervised areas. Before a new room can be authorised for radiation work the Safety Office needs to be informed so that an inspection can be carried out. Only workers formally registered with the Safety Office are allowed to work with radioactive substances, and all work must be carried out under a project code issued by the University Safety Office, following registration of the project. A risk assessment for the protocols to be followed must be posted in the radiation area. Workers are only permitted to work without direct supervision when trained and deemed competent by their supervisor and must attend the University’s radiation course at the earliest opportunity. The laboratory door must be kept closed at all times and locked when it is not in use. Ordering and accounting for radiochemicals All radio-isotope orders must be processed through and approved by the RPS. To place an order you must have a University radiation project code. All new stock must be collected by an authorised member of the laboratory, who must sign for its receipt. This person is responsible for ensuring that it is securely stored and that the RPS is informed so that it can be entered on Isostock; depending on local arrangements, this entry may be made by the authorised user or by the local RPS All new isotopes must be registered on Isostock before use. Primary isotope stocks must then be labelled with the unique identifier prescribed by Isostock. 26 All stock solutions, working solutions and samples (or sample batches) must be labelled. All labels must include the unique identifier given by Isostock to the source solution that you are using. Depending on your protocol, Isostock may ascribe further identifying code numbers to working solutions diluted from your primary stock. All of your solutions or sample batches must be able to be traced back to the primary stock. Together with your identity code all labels must state the solution's chemical form, activity, date, your name, and supervisor’s initials. All isotope stocks must be kept in the designated section of either the refrigerator or freezer as appropriate and must be locked when not in use. Remember to record the amount of radioactivity used and disposed of in your experiment and immediately e-mail this information directly to the RPS or enter the information onto Isostock if you have access rights. Liquid waste may only be disposed via a designated sink and all solid waste must be date labelled before being put into the appropriate waste bin that displays the isotope and month of use. Solid waste bind must be sealed and disposed of at the end of every month. General working procedures Laboratory coats are on personal issue and must be carefully monitored at the end of each radiation session to ensure that contamination is not spread when stored in proximity to others. They must be sent for laundering on a regular basis or if contamination is detected. Good laboratory practice must be followed at all times. Personal dosemeters (body and finger badges) and disposable gloves must also be worn at all times. Dosemeters must be kept away from the radiation lab when not working with radiation, as they might record a dose that you have not actually received. Dosimeters are issued by the Safety Office when registration has been completed. Lost dosimeters are chargeable to the cost centre of the worker. All manipulations of unsealed isotopes must be carried out in spill trays with an absorbent liner (paper towels or 'Benchkote' absorbent side up). Plan your work well in advance, make sure that you have the correct equipment to hand and always work behind the screens and minimise the time of exposure. Remove the radioactive stock from the specially allocated storage arealast and once thawed remove an aliquot for your experiment. Be aware that most spillages occur while opening the stock. The stock should be returned to the freezer immediately after use and should not be left on the bench unless it is necessary to do so. Contamination monitoring Monitor the whole of the supervised area for radioactive spillages before commencing your work and after completing it. Record the monitoring results in the monitoring book, along with the identification number(s) of all monitors used in making the measurements. During your experiment, remember to monitor regularly your hands, equipment and surrounding area for accidental spillages. When you finish your experiment make sure you tidy up, monitor the whole area and clean any spillages. Remember you may be the next user and you will not be happy if the working area is contaminated and messy! 27 Accidents and contingency arrangements Notify the your local RPS immediately in the event of a spill of radiochemicals. Barrier off the area and warn other occupants of the laboratory. Any spillages should be cleared immediately and then decontaminated with a 2% solution of Decon 90. The cleaned area should be monitored for residual contamination and the cleaning process repeated if necessary. A major spillage must be reported to the University Safety Office immediately. Maintenance and cleaning staff Before any work is carried out by maintenance and cleaning staff, the area they are to work in must be cleared of radioisotope and monitored to confirm that there is no hazard present. Written information must be provided on the precautions to be observed. If any radioisotope cannot be secured away, or there is work in hand that cannot be stopped, then a member of the laboratory must supervise the work. COSHH New requirements for the control and handling of hazardous substances in the workplace were introduced in 1999. The Control of Substances Hazardous to Health states that all employers are legally bound to carry out the following for every known biological or chemical hazard or substance: Access the risk to health Introduce ways to control the risk Maintain control measures Monitor exposure of staff if appropriate Inform staff about the risks and implement training. Risk Assessment and hazards Risk assessments must be carried out before work can start in any MOL lab. Information on how to complete these can be found in the Risk Assessment Form and Guidance Notes on the MOL safety web pages. Assessments must be reviewed on a regular basis and updated or deleted as appropriate. Assessments must also be carried out for procedures such as manual handling and cylinder handling or incorporated into SOPs. For assessments that may be required for staff or students with disabilities, input from Occupational Health is recommended. 28 Electrical Safety The School will comply with the University Code of Practice for Electrical Safety. This is accessible at:http://www.nottingham.ac.uk/safety/publications/circulars/#Electrical All items of portable electrical equipment need to be inspected/tested in accordance with the guidance in this document. You must not ‘import’ electrical items into your area unless they are to be tested. Do not use them until they have been tested. D.I.Y. Repairs of electric equipment ARE PROHIBITED. Return items for repair if bare contacts or wires are exposed, or if the cable is frayed, if the fuse blows seek assistance and get the equipment checked. An inventory of equipment must be maintained by divisions. Any new items of electrical equipment, including any brought from home, must be added to the inventory and inspected or tested. Equipment taken from storage, equipment repaired and equipment loaned to (or by) the School must be tested. Electrical testing is only to be carried out by competent suitably trained staff and the records of the testing must be held at divisional level. The categories and frequencies of testing are as follows Annual testing Portable office equipment, portable laboratory equipment, power tools, and other workshop equipment, personal mains powered electrical devices (stirrer’s hotplates, pumps, fans, heaters kettles, etc) Biannual testing Non-portable laboratory refrigerators etc) 4 yearly testing IT equipment including computers; equipment containing microprocessors. equipment (e.g. Ovens, office large equipment, centrifuges, laboratory The term visual testing is taken to mean a formal inspection of the wiring, plug, fuse, and external surfaces of the item. Visual testing should be performed every time a piece of equipment is moved and annually for all equipment in addition to the testing schedule. Any three-phase equipment must be installed and tested by the University's electricians or nominated contractors which must be organised via the Estates Department. 29 Guidance for IT staff servicing PC`s in MOL labs. Before you go into laboratory areas you need to know, what PC needs servicing (and what’s the problem), where is it and when is a convenient time to do it. This is best achieved by talking to the individual requesting the job. This sounds obvious and isn’t essentially a Health and Safety concern; but it does allow you to make decisions about how best to control the situation and minimise risk. The first question to ask from a H&S point of view is, “can this PC be worked on back in the IT suite, or do you need to work on it in the lab? Asking this in advance allows the equipment and area it is in to be made safe before you turn up to do your work. Taking equipment out of the labs Before removing equipment from the labs it must be decontaminated. For Mol this means cleaning to remove potential Biological and Chemical contamination. This needs to be documented using a “Certificate of Decontamination” and you should ask for one; and ask for clarification of what the associated hazard was and how it was dealt with if you are unfamiliar with the hazard. Ideally you should only take the “tower”. As well as probably being the only piece you need to look at, this is the easiest component to clean. Monitors, keyboards and mice aren’t as serviceable and you will have spares back at your desk or need to replace. Ask for the PC and the paperwork to be made ready and removed from the lab ready for you to collect. Working in the lab Even though the equipment is in the lab it needs decontaminating before you are asked to work on it. Again ask for a “Certificate of Decontamination” and ask for it to be explained to you. You should also be given a “Hazardous Work Permit” detailing hazards associated with the room, control measures to be employed where you are working and indicating what PPE you need to be provided with. Returning equipment to the lab When you have serviced the PC you may need to test it on the lab apparatus it controls. If so there is a need to contact the person requesting the work. They need to make the area safe, provide you with a “Hazardous Work Permit”, provide PPE and control that area whilst you are working. Communication The key to controlling work in hazardous areas is communication. The lab staff need to tell outside workers what needs doing and the hazards associated. The outside workers need to ask for clarification of anything they do not understand. 30 Fire Regulations REPORT ALL SUSPICIONS OF FIRE IMMEDIATELY - DO NOT DELAY RAISING THE ALARM. LIVES COULD DEPEND ON IT. All members of the school must ensure they know the positions of the nearest fire alarms, telephones, fire extinguishers and exit routes. These differ within the School but the general rules apply. See relevant area. Get to know the location and identity of the different fire appliances available to you. Do not allow an accumulation of flammable materials (paper, solvents) in uncontrolled areas and ensure that storage of such items is safe and tidy. If asked to evacuate the Building: 1. Do not use lifts or stop to collect personal belongings. 2. Report to the assembly point. 3. Do no re-enter the building until instructed that it is safe to do so. Fire extinguishers: All new fire extinguishers in the EU are painted in signal red and the important colour coding is on their labels. You may find older ones which are solid colours rather than red and whilst these are still legal they will need to be replaced at the end of their useful life. The correct extinguisher to use: Carbon Dioxide (black) Type of fire Water (Red) Foam (Cream) Powder (Blue) Carbonaceous Solids (Wood, paper etc). Yes Yes Yes No Liquids or liquifiable solids No Yes Yes Yes Gases (butane, propane, natural etc.) No No Yes No Electrical hazards No No Yes Yes Fire Lectures: All members of staff are required to attend a fire lecture every year. Local lectures will be posted in each department and an attendance list kept. Any school member of staff must attend a fire lecture at QMC if they use or visit the QMC site and a record of attendance is maintained by the MOL Teaching Facility. 31 Queens Medical Centre The Estates Office tests the fire alarm system each week on Thursday mornings. There are electronic alarm sounders installed in QMC. There are two types of alarm signal within QMC, these are the "two tone" (continuous) alarm signal and the "single tone" (intermittent) alarm signal. A Fire Information Panel is located within each fire zone. This shows the main access corridors and staircases as well as fire zones for that floor. A number and a red light identify each zone. Should one or more of these light up then a fire or smoke detector has been triggered in that area. The exact location of the detector that has been triggered will be given in the digital display at the top of the panel West, South, East & the new ENT Block On discovering a fire: 1. Activate the Fire Alarm by operating the nearest break glass alarm call point. 2. Dial 2222 via the nearest telephone and state location of fire, this is to give further information only and will not set off the alarms. 3. Evacuate the area into the next fire zone, closing the fire doors behind you. 4. Use appropriate fire extinguishers if safe to do so and if your escape route is clear. On hearing the fire alarm: "Two tone" (continuous) alarm: 1. Fire alarm triggered on your floor. 2. Check the fire information panel for the location of the fire. If it is in your area then carry out procedure above. 3. If not located, do not go through the fire doors. 4. Wait for alarm to stop or Fire Officer's instructions to evacuate the area. "Single tone" (intermittent) alarm: 1. Fire in your block above or below your floor. 2. Do not move between floors however movement laterally between blocks is permitted. If you trigger the fire alarm in error: If you know that the fire alarm has been triggered in your area in error (burning toast, dust created by contractors etc): 1. Ring 2222 immediately and inform the switchboard what has happened. Do not wait for the fire team/brigade to attend to inform them. 32 Medical School The Estates Office tests the fire alarm system each week on Thursday mornings. On discovering a fire: 1. Activate the Fire Alarm by operating the nearest break-glass alarm call point. 2. Dial 2222 via the nearest telephone and state location of fire, this is to give further information only and will not set off the alarms. 3. Evacuate the area into the next fire zone, closing the doors behind you. 4. Use appropriate fire extinguishers if safe to do so and if your escape route is clear. On hearing the fire alarm: "Two tone" (continuous) alarm: 1. Fire alarm triggered on your floor. 2. Check the fire information panel for the location of the fire. If it is in your area then carry out procedure above. 3. Collect red token for checked clear area and take down and hang on its predetermined position on the fire token board, which is situated in the A floor foyer next to the main front stairs. 4. If not located, do not go through the fire doors. 5. Wait for alarm to stop or Fire Officers' instructions to evacuate the area. "Single tone" (intermittent) alarm: 1. Fire in your block or below your floor. 2. Do not move between floor however movement laterally between blocks is permitted. If you trigger the fire alarm in error: If you know that the fire alarm has been triggered in your area in error (burning toast, dust created by contractors etc): 1. Ring 2222 immediately and inform the switchboard what has happened. Do not wait for the fire team/brigade to attend to inform them. On hearing the fire alarm in the lecture theatres during a class: If a single tone sounds, no action is required. In the case of a two tone continuous alarm, the lecturer should evacuate the room. For LT1 this should be via the doors into the foyer and then out into car park 1. For LT3 & 4 this should be via the back doors and to the outside via the School of Nursing exit or the goods yard exit. On hearing the fire alarm in the teaching labs when a class is in progress: If a single tone sounds, no action is required. In the case of a two tone continuous alarm sounding the lecturer should tell the students to remain in the room, the lecturer or other member of staff, searches the area and if no fire is found, should take the token for that area down and hang it on its predetermined position on the fire token board, which is situated in the A floor foyer next to the main front stairs. 33 Staff Procedures When the alarm sounds there is a standard response procedure within the medical school. During the working day 1. 2. 3. 4. 5. 6. The fire brigade are called automatically via the QMC switchboard. The medical school engineers will respond. The QMC fire officer will respond. The QMC duty nurse manager will respond. A safety or fire officer from the medical school will respond. QMC security will respond. Out of hours 1. 2. 3. 4. The fire brigade are called automatically via the QMC switch board. The QMC estates shift technician will respond. The QMC duty nurses manager will respond. QMC security will respond. Centre for Biomolecular Sciences On discovering a fire: 1. Raise the alarm using one of the fire alarm points. If practicable dial 8888 on the nearest internal phone and inform security. 2. Close the door and evacuate the area. 3. Use the appropriate fire extinguisher if it is safe to do so and your escape route is clear. On hearing a continuous alarm 1. Pick up fire token and check areas listed on it. 2. Take token to Safety Officer at the fire assembly point located in the car parking area. 3. Await instructions before re-entering the building. City Hospital Nottingham On discovering a fire: 1. Raise the alarm using one of the break glass points or ring switchboard on 2222 and inform them of the location of the fire. 2. Close the door and evacuate the area. 3. Use appropriate fire extinguishers if safe to do so and if your escape route is clear. 34 Clinical Sciences Building On discovering a fire: 1. Smoke or heat detectors trigger alarm automatically. 2. Close the door and evacuate the area. 3. Use appropriate fire extinguishers if safe to do so and if your escape route is clear. On hearing the fire alarm: Continuous siren: 1. Pick up fire token and check areas listed on it. 2. Take token to Safety Officer at the fire assembly point located at the front of the Clinical Sciences Building, next to the barrier to the rear car park. 3. Await instructions before re-entering the building. 35 First Aid and Serious Injury First Aid assistance should be summoned by calling for a departmental First Aider if available, or contacting Accident and Emergency. In Cases of Serious Injury Take person to Accident and Emergency or in the case of Cardiac Arrest use appropriate number to call the resuscitation team. First Aid Boxes First Aid boxes are kept within the divisions and you should familiarise yourself with these within your own unit. The First Aiders maintain any supplies and any use should be reported to them. Chemical Contamination. In the event of chemical contamination the procedure is as follows: Eyes: Irrigate thoroughly with water and/or sterile infusion saline (0.9%w/v sodium chloride) for at least 10 minutes. If discomfort persists obtain medical attention. Water can be applied to the eyes via tubing attached to cold water taps in laboratories. Lungs: Remove from exposure, rest and keep warm. In severe cases obtain medical attention. Skin: Wash skin thoroughly with soap and water. Remove contaminating clothing and wash before re-use. In severe cases obtain medical attention. Mouth: Wash out mouth thoroughly with water and give plenty of water to drink. Obtain medical attention. Spillages:All blood spillages, no matter how small, must be mopped up immediately using tissue soaked in Trigene, the contaminated tissue being disposed of in the yellow clinical waste bags. Accident and Incident Reporting All injuries, accidents and dangerous occurrences must be reported to divisional safety officers (or in the event of their absence to the School Safety Officer directly) who will report the incident using the University on-line reporting system which can be accessed via the safety office web site. The University Safety Office must be contacted immediately following major injuries and certain dangerous occurrences. This is carried out through the School Safety Officer or divisional reps if appropriate. 36 Manual Handling Operations Under the Manual Handling Operations Regulations 1992, you should avoid hazardous manual handling operations if possible. If this is not possible then a suitable assessment should be made of the operation and the risk of injury from that operation should be reduced so far as is reasonably practicable. The School Safety Officer can arrange training on manual handling procedures from the Occupational Health Unit. School Safety Audit To ensure that the School’s policies and procedures are being correctly implemented, the School Safety Officer and the local divisional Safety Officer will carry out inspections annually. The frequency of monitoring will depend, to some extent on the issues involved. Issues arising from inspections will be reported to the Safety Committee and appropriate remedial action instigated. A list of audit/inspection standards is included at the end of this document for guidance. Any member of the School should report any defects, faults and other safety hazards to the Divisional Safety Officer or directly to the School Safety Officer as soon as possible. The school safety manual will be regularly reviewed and the on-line copy amended accordingly. 37 Out of hours policy The working day is defined as 8.00 to 18.00 from Monday to Friday and it is assumed that the majority of staff and students will be at work during these hours. Some work may be required to take place outside of these hours and must be assessed using guidelines below. A notification to work out of hours form must be completed along with a risk assessment for consideration by the appropriate academic supervisor. Policy on Working Outside Normal Hours for MOL Laboratories. From the University Safety handbook ‘6.4 Out of HoursWorking’ Some Schools are open outside normal working hours for experimental work. The potential dangers of carrying out experimental work alone at night are much greater than normal and this practice should be strongly discouraged. Any practical work out of normal hours must only be carried out with the knowledge of the School Safety Officer and the consent of the Head of School. If such work is essential, every effort should be made to ensure that another worker is within hearing distance and the Security Staff should be asked to make regular contact. To facilitate this it is essential that any out of-hours register or equivalent is filled in on entry AND departure from the School or building. General study facilities out of normal hours should be concentrated in specific areas to ensure the control and safety of persons involved. Notices to advise on the action to take in case of emergency should be posted in 24-hour access computer rooms. Context Should an accident occur during this period it can be reasonable to expect that there should be assistance close to hand to deal with the situation. Staff and students are encouraged to organise their work to take place during normal working hours as far as is reasonably practicable. However, there may be a valid need to work outside of these hours and at weekends when the numbers of staff may not be adequate to deal with any emergency situations arising. All divisions must operate a restricted out of hours access policy to provide a safe working environment that includes completion of the out of hours form and risk assessment as described in this document. The need for students to undertake work outside of normal working hours must be subject to tight controls. First year Post graduate students registered for Phd`s may be eligible to undertake such work if they demonstrated the required competency and the assessment formally recorded on the training forms. The work that is being carried out must also carry a low risk assessment score. Under no circumstances are MSc and BMed students be allowed to work outside of normal working hours unless under the direct supervision of their own supervisor who must be present in the area of work with them. 38 Risk Assessment Risk assessment is the key to reducing accidents. During extended periods of work reduced staff and student numbers may not increase the likelihood of accidents occurring but the swiftness of response will be compromised and the value of risk assessment is enhanced. Accordingly, risk assessment is one of the key areas to ensure a comprehensive and workable out of hours policy within the School. Further guidance on risk assessment and how to complete the form can also be found on the MOL safety web pages. Buddy System The second area of importance in the out of hours policy is the buddy system. Risk assessment should be used to identify the need for a co-worker to be present and able to respond in the event of an accident or injury occurring when work is being undertaken outside of core hours. Practical arrangements Staff and students wishing to perform work out-of-hours need to satisfy several criteria to ensure their personal safety and the security of the equipment in our labs. Duties of the individual worker proposing to undertake work out of hours include: ● Identify the need to perform work out of hours. Prepare / locate a risk assessment of that work. ● Timetable the work. Identify that the planned work can be completed and the work-area made safe. ●Arrange a contact person to notify when entering the department and leaving the department. The contact person should check up on the worker if they have failed to get in touch on leaving the department at a pre-arranged leaving time. If contact cannot be made, QMC / University security should be contacted immediately to investigate a potential accident. ● If the risk assessment indicates, identify a second individual, conversant with, competent and trained in the planned work. Secure their agreement to stay within reach during the entirety of the timetabled experimental period. Planned work involving reagents with a potential to incapacitate (e.g. certain chemicals, or working with a Bunsen burner etc) demands a buddy. Prior to the first occasion of undertaking a particular technique out of hours, present risk assessments to your supervisor or the local safety representative together with the completed “Permission to work out of hours” form detailing the work intended, the designated contacts and the buddy’s agreement. Duties of the person agreeing to maintain contact with staff and students working during the extended period. ● To be willing to receive phone / text / e-mail contact from the individual conducting the work. ● To check the chosen means of contact at an agreed time to determine the working individual has safely left the department. 39 ● Raise the alarm with an appropriate body if the worker has not made contact within the prearranged timeframe. The contact must therefore be equipped with all relevant local emergency numbers. Duties of the buddy agreeing to accompany those working out of hours: ● To assist in the risk assessment process in order to satisfy the requirement that the buddy is a suitable candidate. ● To be willing and able to accompany the worker for the timetabled period. ● To remain within assistance of the worker. If the buddy is occupying a neighbouring lab or office there is a requirement to periodically check on the progress and welfare of the worker. ●To remain in visual contact and be prepared to assist during manipulations that a risk assessment identifies as particularly hazardous. Duties of the supervisor agreeing to authorise staff and students working during the extended period: ● To confirm the need to perform the work out of hours. An issue here is whether it is essential for the work to be performed at that time, or it is convenient for the researcher and their buddy that it be performed at that time. ● To approve the risk assessment and make a judgement on the score given to the timetabled work. This could be done for an extended period of say a month if all the work was in the same risk category. ●To confirm the suitability of an individual or a list of staff suitable to act as a buddy as they relate to the work timetabled. Duties of the worker and buddy working out of hours: Before 7.00 in the morning, after 19.00h during the week and at weekends, both worker (and where appropriate, the buddy) are required to complete the “Out-of-Hours” book or forms held locally. By indicating name of workers, room numbers occupied, intended work, and grade given by risk assessment of the work performed, in the event of an emergency requiring evacuation, the out-of-hours log will be used to determine occupancy. Upon leaving, both worker and buddy are required to sign-out, logging the time of leaving. It is an absolute requirement that books or forms be completed upon exit. General Advice and Exceptions Risk assessment may reasonably identify office-bound and many laboratory procedures as acceptable to perform out of hours, when the likelihood and consequences of a potential accident are low and infrequent. Staff and students engaged in such work may be excused from the buddy system and the responsibilities of individuals working alone or in low risk activities can be found on page 41. The requirement to complete the “Out-of-Hours” form remains. Accidents can occur in office activities so it is good practice to inform others off-site of your intentions similar to the formal procedures outlined for laboratory work. 40 Risk assessments with high scores may demand supervision that may not reasonably be provided outside of normal working hours. It may be prudent to decide that such risks are not acceptable during these times. Should essential work need to be completed during the out of hours period, advice should be sought from the local safety representatives, the School Safety officer or Building Safety Officer. First-year postgraduate students and any other inexperienced laboratory workers may not plan work out of hours that carries a high risk assessment score without the use of a buddy. The position of visitors to our labs will depend upon their background, previous training and the completion of documents confirming assessed competency. Forms should be returned to the local Safety Officer by at least 4pm on the day work is performed. Again, this will assist in the evolution of this policy to achieve the balance between our twin commitment to health and a productive research environment. NOTE:UNDER NO CIRCUMSTANCES IS LIQUID NITROGEN TO BE HANDLED OUT OF HOURS WITHOUT A BUDDY. 41 RESPONSIBILITY OF INDIVIDUALS WORKING ALONE IN OFFICES OR LOW RISK AREAS/ACTIVITES LW must ensure that any medical conditions which might be relevant to your working alone are fully discussed with their line manager and, if necessary, Occupational Health and own GP. Individuals must not work alone if any such condition is assessed as placing them at increased risk. Any person who requires assistance to get out of the building in an emergency must not work alone. The LW must comply with the out of hours log in/out arrangements in operation within the building and it is strongly recommended that they set up end of work contact arrangements with a family member, friend or colleague. Any person authorised to be in the building outside normal hours must be fully competent to carry out the work safely and be fully conversant with emergency procedures. Access to the building is restricted to authorised personnel outside normal hours. In the event that the LW has concerns about security or suspects there is an intruder in the building they must contact Trent Security on ext 13013. Do not confront the intruder, lock door and await arrival of TS. The LW should plan how to get to car/public transport after leaving, taking account of potential personal safety issues. LW should consider use of personal attack alarm. LWs must know local arrangements on how respond in event of fire or other emergency First aiders are unlikely to be present. First aid boxes are available and contents checked regularly. In the event of a LW feeling unwell they should if possible return home or contact Security on 13013 for assistance. LW should be aware that heating/cooling in the area may be much reduced unless the business need for after hours working has been established. Report any hazards in offices [e.g. torn carpets, uneven flooring, trailing cables etc] to a responsible person so remedial action can be taken Individuals with temporarily impaired mobility must not work alone. LW must not interfere with electrical plugs or power supply. 42 Notification of intent to work out of normal hours. (before 7 am and after 7 pm Monday to Friday; all weekend and bank holidays) Name: Supervisor………………………………………………………………………… Part I: to be completed by worker My research requires that I complete the following tasks out of normal working hours: I have timetabled this work to be completed and work-areas made safe on …./…../….. I have completed the relevant Risk Assessment and been certified as competent in the techniques involved: Risk assessment score………………………… I have asked…………………………………………………to be my contact person. I will make contact with the above when I arrive at work and tell them when I plan to leave work. I will make contact again when I’m about to leave / have left work. They are aware that if they don not hear from me within this agreed timeframe and cannot contact me, they must call either QMC or University security immediately. I have given them emergency contact numbers for this purpose. Signature:…………………………………………………. Date: …………………………… Printed name: ………………………………………….. Laboratory number: …………………………………………………………………………………….. Part II: to be completed by project supervisor I agree that (name)………………………………….. needs to complete the work at the time and date specified; and that she/he has completed the relevant risk assessments and has been certified as competent in the techniques involved: Signature:…………………………………………… Date: ……………………………………….. Printed name:.……………………………………………………………………………………………………. 43 Supplementary form to be filled in where risk assessment dictates a coworker: Part III: to be completed by the buddy who has agreed to remain in contact during the timetabled work I have agreed to accompany (name) …………………………………..from hrs until ……….hrs on …./…../…. Signature:……………………………………………… Date: ………………………………………… Printed name:.………………………………………… Laboratory number: …………………. …………………………………………………………………………………….. 44 New and visiting workers and students The School has a responsibility to ensure a safe working environment for all staff and students. Policy for Workers and students invited to work in Mol labs The School has a responsibility to ensure a safe working environment for all who come to work in the building. Permanent members of staff and registered students must receive a safety induction and complete an appropriate training record recording training activities and competency. To assist in this process the local and school Safety Officers must be given sufficient notice of the arrival of such workers and the form on page 45 completed. Information on the form will identify the need for training, competency assessments and risk assessments. Identification of the introduction of new working practices or any chemical or biological material that they may be bringing with them can also be identified in advance and the arrangements to receive such material put into place. University staff and students using facilities in Mol labs. Staff from other schools may wish to use the facilities and equipment Mol provides. Such equipment should have a standard operating procedure and risk assessment and the individual responsible for supervising the facility/equipment must ensure that visiting staff receive a copy of these and complete the training component of that assessment. Additionally, the visitor should bring appropriate PPE or be provided with the required items. The visitor should also notify the lab manager/supervisor of any new hazards needing to be brought into any MOL lab and have made risk assessment of them, including provision for containment, storage and transportation of material. 45 Pre-notification of workers wishing to perform research in the School of Molecular Medical Science Staff member and worker nominated Name of Member of University: Department: Name of visiting workers: Organisation of visiting worker: Duration of work (dates from/to): Location of the laboratory/office Work to be performed Brief description and justification of work to be carried out by visiting worker Once completed, please send the form to Di Mitchell and Julie Stanley 46 Supervision, training and arrangements How? Who will be supervising them? Who is nominated as a secondary/bench supervisor? Where will they be working? What is their competence in the necessary lab skills? How has this been assessed? Who will be providing training? Will they require permission to work out-ofhours? What? What hazardous substances will they be bringing into Mol? What hazardous substances will they be taking out of Mol? Where will it be stored? How will this be transported? Signature: Name: Date: 47 Children, young persons and adult companions on University Premises, assisting in University Work. The School will comply with the guidelines set out in by the University. These are summarised in the University Safety Handbook. Maintenance workers and company representatives Labs are hazardous areas and must be made safe for workers entering them in accordance with the University policy: Policy on maintenance workers and company representatives University estates workers, external contract workers and company technical specialists and representatives often need access to Mol labs to install, repair or maintain equipment and facilities. All such visitors should sign any visitors book held locally indicating name, company, contact staff member, location and nature of work and arrival time. The member of staff arranging for such visits should be available to meet the visitor and nominate a deputy to be available in their absence. Labs are hazardous areas and should be made safe for workers entering them in accordance with the University policy on entry into laboratories, workshops and other hazardous areas: www.nottingham.ac.uk/safety/publications/circulars/HazAreaEntry.doc. “The Permit to Carry Out Maintenance Work in a Hazardous Area” should be completed indicating to the worker that the area has been made safe and identifying the requirement to stop work in that area, and provide PPE and supervision. Equipment should be decontaminated before service personnel can begin work on it. This is documented using the “Certificate of Decontamination” (see below), a copy of which should be given to the maintenance operative. Work performed which may interfere with the fire-alarm system will require that local sensors be deactivated, this should be arranged in advance with Estates and a “Hot-work permit” completed. All such forms are held with the local Safety officer, to whom a copy should be returned. Equipment should be decontaminated before service personnel begin work. This is documented by use of the “Certificate of Decontamination” Laboratory Equipment Used with Hazardous Substances 48 Certificate of Decontamination This form is to certify that equipment that might have been contaminated with hazardous substances has been decontaminated before repair or disposal. You must know all of the substances that have been used and produced in the equipment before you complete this form, which must accompany the equipment for repair/removal Section 1: Equipment Equipment model number Serial Number Plant Number Usual Location Section 2: Substances In Contact With The Equipment Are any of the substances used or produced in the equipment: Biologically Active Dangerous to human health or safety Radioactive N.B If the item is potentially radioactively contaminated, proof of decontamination must be supplied. Section 3: List of Substances In Contact With The Equipment Substance name or type Associated Hazards Section 4: Method of Decontamination Section 5: Declaration Date of Decontamination Carried out By ( Print Name) 49 Job Title Contact Details Signed Date 50 Immunisation Before commencing work with human/animal tissue or genetic manipulation you are strongly advised to undergo a course of Hepatitis B, Tetanus and BCG vaccination. The Occupational Health Unit can check your immune status and carry out the above immunisations if required. Allergen Screening Allergen screening tests are essential for people working in the Biomedical Services Unit. An appointment for screening must be arranged by contacting the Biomedical Services Unit on Ext 30060 prior to any work commencing. 51 New and Expectant Mothers If you become pregnant during your employment you must inform your line manager and safety officer. Information will be provided to new or expectant mothers in relation to work activities and processes that could adversely affect them or their unborn child. The risks may be physical (e.g. manual handling, ionising radiation), biological (e.g. contact with blood and body fluids, micro-organisms, or chemical (e.g. carcinogenic substances). The following is taken from the Safety Office Policy P2/99A and is in line with current European Directive. New and Expectant Mothers at Work The Management of Health and Safety at Work Regulations include the duty upon employers to protect the health, safety and welfare at work of any new and expectant mothers in their employment. This information below describes the University’s arrangements for compliance with these regulations and is relevant to both employees and students. The guidance is in line with the European Directive on Pregnant Workers and refers to other sources of information. 1. Definitions: New or expectant mother - a worker who is pregnant, who has given birth within the previous six months or who is breastfeeding. Given birth - delivered a living child or, after 24 weeks of pregnancy, a stillborn child. 2. Responsibility of the Head of School (or equivalent) It is the responsibility of the Head of School to ensure that there are arrangements in place for the identification of those activities and processes that could present a risk to new and expectant mothers at work and for providing appropriate information to female workers of child-bearing age. A general statement that identifies generic processes and activities and describes the procedure to be followed in the event of a pregnancy should be published in the School Safety Policy. 3. Responsibility of the Individual Notification of Pregnancy Where the nature of the work area or work activities may present a risk to a new or expectant mother it is the responsibility of that person to notify the School at the earliest opportunity in order that appropriate action to remove or adequately control the risk may be taken. The Head of School may at that stage require written confirmation from her medical adviser. In all circumstances it is the responsibility of the individual to notify the Personnel Office of pregnancy in accordance with the University's Maternity Leave Regulations, ie receipt of a 'Mat B' Certificate 13 weeks before the expected date of confinement. 4. Risk Assessment A summary of known hazards that may affect the health and safety of new or expectant mothers is given in Appendix 1 ( see below ) Once an individual declares herself pregnant her manager must ensure that a more detailed assessment of the risks from the work activity is carried out. Refer to Appendix 1. This assessment must also consider the activities of other workers in the same area 52 since these may also affect the health of the mother or foetus. Any risk must be reduced to an acceptable level. It is preferable to remove the risk and if this is not possible, the risk must be controlled. Within individual work areas, information should be provided on those processes which could present a specific risk to new or expectant mothers. If unacceptable risks to the safety of the new or expectant mother at work remain, steps must be taken by the School to remove the worker from the risk. The following options may be available and should be discussed in liaison with the Personnel Office and Occupational Health: o Temporarily adjust the working condition and/or hours of work of the worker o Offer the worker suitable alternative work if any is available, if not o Suspend her from work (on the full normal rate of remuneration) for as long as necessary to protect her safety or health or that of her child. (This option will not affect the individual's maternity rights, pension rights or length of service.) If there are concerns regarding the medical aspects of pregnancy or the risks involved, advice may be sought from Occupational Health or the Safety Office. In exceptional circumstances, Occupational Health will liaise with the individual's general practitioner or obstetrician. The risk assessment should be recorded in the same way as any other risk assessment in the School. Alternatively a model form is provided in Appendix 2. 5. Other considerations Aspects of pregnancy that might affect work In addition to risks presented by the work activity itself, there are aspects of pregnancy that may impact on the way the individual is able to work. Such aspects including sickness, backache, increasing size, frequent visits to toilet, tiredness, dexterity, agility, balance and comfort. Managers must give consideration to these aspects as circumstances dictate. Changes of work activity or the way in which an activity is carried out may be required. 1. Rest at Work Pregnant workers may, at times, suffer from fatigue and other effects, especially during the latter months of the pregnancy. If an expectant mother is in need of rest during the working day, she should be permitted to sit in a suitable and quiet area of the building, e.g. office, vacant meeting room, café, library, etc. Where the need for regular rest periods has a significant impact on work, the Head of School, through Personnel, may request an assessment by Occupational Health. This could include whether sickness leave or statutory maternity leave should commence. Further information concerning absence or sickness related to the pregnancy is contained in the University's Personnel Policy relating to Maternity Leave. 2. Breastfeeding There is no fixed time span for breastfeeding and it may vary considerably. During breastfeeding, the worker must not be exposed to risks that could adversely affect her health or that of the baby. The worker should inform their manager that they are breastfeeding and advice may be sought from Occupational Health. More information on the risk to breastfeeding mothers is given in the publications listed below. 53 3. Nightwork Consideration should be given to new and expectant mothers who work at night. Specialists are not aware of any risk to pregnant or breastfeeding workers or their children from working at night per se. However, the individual's medical adviser may decide that working at night is to be avoided on the grounds that they consider the worker’s health or safety would be adversely affected. In the event that nightwork is considered a risk (medical certificate required), the worker should be offered suitable alternative daytime work or suspended on full pay for the pregnancy term. 6. Summary of actions required: 1. Incorporate arrangements for this issue in the School Safety Policy 2. Follow School risk assessment procedure to include specific risks to new and expectant mothers 3. Implement measures for control of risk where necessary 4. Provide appropriate information to women of child-bearing age 5. Upon being notified of pregnancy, carry out specific risk assessment to identify any additional risks 6. Implement any further control measures required or remove worker from risk 7. Ensure that worker is not at risk if breastfeeding on return to work 8. Review arrangements periodically to ensure valid Published Guidance The following publications are available in the University Safety Office: Management of Health and Safety at Work Regulations 1999 New and Expectant Mothers at Work - A Guide for Employers, HSG 122 (2002) A Guide for New and Expectant Mothers Who Work - HSE, INDG 373 - For employees. (http://www.hse.gov.uk/pubns/indg373.pdf) Infection risks to new and expectant mothers in the workplace - A Guide for employers (HSE Advisory Committee on Dangerous Pathogens 1997) Hazards for Pregnant Nurses: An A-Z Guide (Royal College of Nursing, 1995) New and Expectant Mothers at Work - a Guide for Health Professionals (HSE, March 2003) (http://www.hse.gov.uk/pubns/indg373hp.pdf) COSHH Regulations 1994 Workplace (Health, Safety and Welfare) Regulations 1992 [The School of Nursing and Midwifery has a procedure applying to student nurses who become pregnant and are due to go out on placement.] 54 Summary of known hazards which may affect the health and safety of new or expectant mothers Physical e.g. Shocks, Vibration or Movement Manual Handling Noise Ionising Radiation (specific dose limits for abdomen) Non-ionising electromagnetic radiation Extremes of heat or cold Movements and postures (travelling, standing for prolonged periods, mental & physical fatigue) Work in hyperbaric atmospheres Biological Agents Contact with: Human blood & body fluids Infected animals Laboratory cultures Water or food contaminated by human/animal faeces Bacteria e.g. Brucella, Chlamydia psittaci, Listeria monocytogenes Viruses e.g. Human immunodeficiency, Rubella, Varicella-zoster, Parvovirus, Hepatitis A, Hepatitis B Protozoa e.g. Toxoplasma gondii Chemical Agents e.g. Substances labelled R40, R45, R46 R61, R63, R64 Mercury and Mercury derivatives Cytotoxic (antimiotic) drugs Chemical agents of known and dangerous percutaneous absorption (may be absorbed through the skin) Carbon monoxide, lead and lead derivatives Working Conditions Facilities: Access to resting facilities, hygiene facilities and storage facilities (for expressing and storing breast milk) Hours: Long working hours, shift work and night work may lead to mental and physical fatigue. Occupational stress: Stress may become a problem for various reasons: Hormonal/physiological and psychological changes. Financial, emotional and job insecurity. Difficulties in organising work and private life. Anxiety about the pregnancy or its outcome. Passive smoking: Cigarette smoke is carcinogenic and mutagenic. The University Policy 55 on no smoking should ensure that new and expectant mothers are not exposed to passive smoking whilst at work. Extremes of temperature: Heat stress, sudden changes in temperature, heat dehydration may impair breastfeeding. Display screen equipment: HSE and National Radiological Protection Board advise that levels of electromagnetic radiation generated by DSE do not pose significant risks to the health of mother or baby. Working alone: Pregnant women are more likely to need urgent medical attention, particularly in later stages of pregnancy. More detailed information on the above factors is contained in the second and third publications listed on the previous page. Work with Display Screen Equipment (VDUs) This activity is not specifically listed in the Pregnant Workers Directive. However, in the past, there has been concern about radiation emissions from display screen equipment and possible effects on pregnant women. Research by the National Radiological Protection Board has shown that these concerns are unfounded and no special protective measures are needed to protect workers. To avoid problems which may be caused by stress and anxiety on this issue, Schools should give women who are pregnant or planning children the opportunity to discuss their concerns with Occupational Health. Risk assessment for new and expectant mothers 56 - see safety office web pages. School of Molecular Medical Sciences Staff, student and visiting workers Induction and Training Record Division Name Course if applicable Project title: Academic supervisor: Bench supervisor: A B C Activity Written test Oral test/ discussion Practical demonstration by the trainee. Written documentation received Trainer signature and date Induction to include local safety rules, School safety manual, fire, firstaid, accident reporting, GLP etc. Completion of questionnaire. Waste Disposal routes Induction in agarose gelroom usage Use of Microbiological Safety Cabinets Storage and archiving of research materials to include tissue blocks and patient samples Use of COSHH databases Principles of Risk Assessments and how to complete Ordering procedures and receipt of goods Liquid nitrogen dispensing & handling Specific activities 57 Staff/Student signature and date Competence assessment and how achieved. Date and signature of assessor School of Molecular Medical Sciences Training record for experienced research worker This form to be used to record assessment of competency relating to experienced research workers which may be done by use of the following: A B C Written test Oral test/ discussion Practical demonstration by the trainee. Name Position Start Date Department/area/lab Supervisor/Line manager Deputy Supervisor/Line Manager Project title (Students only) Activity/Procedure/ Course Written procedure/documents received (Y/N) Training Received y/n (Trainee’s signature) Attendance at Postgraduate Health & Safety Induction [ by Safety Office] Attendance at Fire Safety talk [ by Safety Office] Attendance at Biosafety talk [ by safety office] Attendance at radiation safety talk [ by safety office] Local Building Induction for staff/visitors Laboratory safety induction 58 Method by which competence assessed [A, B, C] Required competence attained (Trainer’s name & signature and date ) Add other training courses and key competencies as required e.g cryogenics, good microbiological techniques, specific procedures etc. 59 Audit/inspection Standards Checklist Risk Assessments/Documentation Are assessments available for Risk assessments should be accessible to staff in the activities undertaken /hazards laboratory, Best practice would be to have an area where all encountered in the areas safety documentation is kept and that lab staff know where it is. The folder should be so organised that assessments and other documentation can be easily accessed. GM All work involving the production, use, storage, transport & disposal of GM organisms must be covered by an assessment that has been approved by the local GMSC. This includes already modified organisms imported form outside the university. Areas where GMOs are used should have a local code of practice to cover activates within the area - e.g. where & how the GMO,s used in the area can be handled, grown, stored and details of disposal and disinfection regimes. COSHH Work involving the use of chemical substances, allergens and biological agents [ non modified] must have risk assessments. The approach for chemical substances in many areas is to have hazard assessments for the substance and then a risk assessment for the process in which it is used. This should result in the production of a SOP - which should be appended to the RA. When assessing if they are suitable and sufficient ensure that the control measures follow the correct hierarchy. Radioactive sources Work with radiation sources has to have been subject to risk assessment and approval by the safety office. In addition to RA documents there should also be a copy of the Local Rules & the Environment agency authorisation in the radiation area. There should be documented evidence of recording usage, disposal [ ISOSTOCK] and environmental monitoring. Liquid Nitrogen RAs should address the hazards of asphyxiation, cold burns, manual handling and transport. UV sources Lab gases DSE & Office activities manual handling activities RAs should address the hazards skin and eye exposure of both the operator and of others in the area. There is a guidance document on SO web site which can be used to produce a local SOP - check for evidence of this in the area. RAs should address the hazards of stored energy/pressure, the gas [toxic/ashyxiant/flammable] , manual handling and transport. All work stations used by individual users should have a DSE RA checklist completed - multi user PCs and ISCRAS should have evidence of suitable information to users about correct set up and a generic RA. Any activities that involve the movement of significant loads on a regular basis should have a RA that addresses the load, individual, task and the environment where the activity takes palce. Control measures should consider provision of a mecahnical aid to lift the load or reducing the load where practicable and not just focus on training as the main control measure. 60 Are they reviewed periodically Are they suitable and sufficient for the activity/hazard Are they supported by written procedures where appropriate Are they readily available and do staff know where to access them? TRAINING Are staff/visitors inducted in the area? Are staff/visitors instructed and trained in safe use of equipment and other safety procedures. Assessments should be reviewed periodically and if procedure changes. Frequency will depent on whether the frequency with whihc the procedure changes and the nature of the hazards and risk involved. High risk require more frequent review than low risk. Look for evidence of assessment being reviewed on a regular basis. RAs should identify key hazards in the process and assign suitbale controls to minimis risks. SOPs should be avaiolable for more complex and higher risk activities/procedures RAs should be readily accessible -signed hard copy in lab or easily accessible via computer. Ask staff in lab where they are kept. There should be documented evidence of staff receiving training in emergency procedures and general lab procedures [ e.g. disposal] Individuals should be given job specific training by a competent individual - Ask staff in lab about their training to check this being achieved Are records of training maintained where appropriate General conditions Is area apparently well maintained Training & attainment of competence should be documented. Both trainer and trainee should sign document Are corridors and circulation routes clear of obstruction? Check to ensure that there are no items of furniture or boxes impeding corridors. Circulation areas in labs should be free of trip hazards Are emergency exits clear? Fire doors and fire exits must not be obstructed. Doors should not be wedged open other than with a proprietary door holding device. Areas should be uncluttered and occupancy numbers should not be such as to present a hazard or obstruct circulation routes etc. Is there adequate workspace for the numbers of staff/activities undertaken. Are standards of housekeeping acceptable. Is space used properly Are there any temperature /ventilation problems/ Check the general fabric of the building - any evidence of poor maintenance. Waste bins etc. should not be overflowing. HWB should be clean and there should be soap and towels available. Floors should be at an acceptable level of cleanliness - if not check on cleaning regime. Does the area seem over crowded [ too many people to operate safely]. Is there any unsafe storage of materials at high level. Problems may be obvious at time of inspection but it will also be necessary to ask staff in the area. Materials/substances [Chemicals, BAs, GMMs, Radioactivity] Chemicals 61 Fume cupboards Balance/prep areas Storage of toxic chemicals/carcinogens Flammable storage Acids Labelling ETHIDIUM BROMIDE DISPOSAL of Et Br FCs should not be cluttered with items of equipment/substances not in current use. There should be no items near the front or on the front aerofoil that could impede the sash being shut in an emergency. These should be no items close to or in front of FC that could impede airflows [ see recommendations of BS 7528]. FCs should be serviced and airflows tested annually - this should be documented on the FC itself and service sheets should be available for inspection. There should also be documented evidence of interim checks on airflow at suitable frequencies by lab staff [ using vane anemometer] Should be clean and tidy - no evidence of unidentified powders on or around balances. Weighings should be recorded. If toxic chemicals and carcinogens are dispensed this should be done in an enclosed facility [ FC or Weigh safe] Should be in a locked storage facility In spirit cabinet - ideally vented under a FC. No more than 50litres in the room. Quantities on benches should be <500ml and have hazard label. NO ACIDS/oxidising agents. Check dates on di-ethyl ether and other agents that can generate peroxides. Is there some procedure of recording date a bottle is first opened and of ensuring it is not kept beyond the recommended date. Is BENZENE stored - if so ask for justification of use. Ideally in vented cabinet - no flammables. Any decanted bottles of acids/alcohols etc should have appropriate hazard sign Powder should not be in use. Obtain in concentrated liquid [preferred] or table form. Use in defined areas, contained within trays. There should be specific arrangements for disposal of waste. Check for obvious signs of contamination in areas deposits on visors and screens. There should be regular cleaning as part of housekeeping routine. There should be specific procedures for disposal of chemicals that fall into the category of hazardous waste - this would include Eth Br. Biological/GM Bench handling areas. Handling areas should be clear of clutter - no evidence of papers/pens. Ideally use of trays to contain spills. Benches should be impervious and seals around sinks etc of good quality. Racks should be available and in use to hold tubes etc. Plates and cultures should be secure and not at risk of being knocked over. e. 62 MSC and unidirectional laminar flow hoods [ULF] Growth facilities Storage conditions Labelling DISPOSAL Are there arrangements for safe disposal? Biological/GM material Chemicals/solvents Radioactive materials SPILLS Are there arrangements in place to deal with spillage? Evidence of safe use of: UV sources [SOP, PPE] MSCs should not be cluttered, night doors should be in place if not in use. Bunsen burners should not be used. Alcohol must not be sprayed in cabinet as this may generate explosive atmosphere. MSCs should be serviced and airflows tested at least annually - this should be documented on the MSC itself and service sheets should be available for inspection. There should also be documented evidence of interim checks on airflow at suitable frequencies by lab staff [ using vane anemometer]. ULFs must not be used for growth of any hazardous [ ACDP/ACGM 2] material. Check what they are used for. Signs to that effect are a good idea if there is a mixture of hazards in the room. Incubators - should be clean with no evidence of untreated spillage. Segregation of organisms /cell lines of different hazard groups. Culture to be clearly labelled as to content and so ownership can be verified. There should be a regime in place for regularly checking and cleaning facilities. Wherever possible dedicated fridges or freezers to be used, or have designated shelf . Double containment and use of racks for liquid cultures in tubes. Plates in secure stacks. In cold rooms separation of viable material/cultures from 'clean' items. Use of secondary containment . For full details see University COP. There should be a regime in place for regularly checking and cleaning facilities. All biological agents should bear name of organism/strain/date and owner. General : It is good practice for there to be information about the local lab disposal arrangements & procedures available in the lab e.g. a wall chart. There should be fresh [in date] solution of the disinfectant [ normally Trigene/Virkon] available. Pipette steeps & immersion baths should contain sufficient disinfectant to ensure good contact. Autoclave waste to be in leak proof containers with safe means of transport from lab to autoclave. Procedure for ensuring cycle has completed. Flammable solvent waste should be safely stored [see above] and separated into halogenated and non halogenated. Quantity will contribute to the 50l limit. There should be regular collections and safe transfer to external store. There should be specific procedures for disposal of chemicals that fall into the category of hazardous waste. Refer to local rules. Check that disposal is recorded via isostock Is there a spill kit available ? If not should there be or are quantities such as to not warrant one. There should be an SOP adjacent to the equipment. [available on SO web site] Ideally source should be in cabinet and door interlocked with the UV light. Where open sources are used they should be positioned to prevent accidental exposure of others in the lab. There should be UV opaque visor available clearly labelled for use with UV. There should not be any evidence of dried on buffer on visors or other Perspex screens. 63 UV source should have correct hazard warning signs. Microwave [SOP, PPE] There should be an SOP adjacent to the equipment. [available on SO web site] It is good practice for there to be times and power settings for items that are regularly microwaved There should be face visor and thermal gloves available. Autoclave [SOP/ AU/ PPE] There should be an SOP adjacent to the equipment and a list of authorised users. There should be face visor and thermal gloves available. Autoclaves should be subject to regular checks under the Pressure Systems Regulations - this is carried out by Allianz on behalf of the UoN. Check that this has been done. It should also be subject to regular validation and calibration by a competent person - this is mandatory for an autoclave used to inactivate biological waste. Check that this has been done to the required frequency. rooms containing large quantities of LN may require Oxygen monitoring - if this is not in place ask to see the risk assessment that concludes it was not required. If Ox monitoring in place is the equipment serviced and checked in accordance with the manufacturers recommendations. Pressurised dewars fall under Pressure Systems Regs so require annual check [ see above]. Thermal gloves and visors to be available. If Dewar's have to be lifted manually there should be a manual handling assessment. Ideally cylinders should be located in external stores. If internal they must be secured by rings or chains. Regulators should be inspected annually and replaced every 5 years unless risk assessment determines a different frequency. A list of those authorised to change regulators should be adjacent to cylinders. Manifolded systems may be subject to Pressure Systems regs see above. Flash back arrestors should be used for flammable gases. Regulators should be compatible with the type of gas. Check bowl and buckets for any evidence of corrosion or contamination. There should be a regime of regular cleaning. On large and high/ultra speed machined rotors should be inspected annually by a competent person [often service engineer] so there should be a service contract in place. There should be written procedures on what to do in event of a centrifuge accident/imbalance. Liquid Nitrogen [O2 Mon, PPE] Cylinders [AU list, regs insp] Centrifuges - clean? Rotors insp Work conditions Are noise levels within safe limits? Are there adequate and correct safety signs? Are electrical appliances checked regularly - give date of last check. If it possible to hold a conversation easily with a person a few feet away levels are probably OK. If not a more formal noise assessment will be required . Any safety sign should be a combination of words and pictogram. Words alone are not acceptable. Check dates on PAT stickers. There should be formal arrangement sin place to ensure new equipment does not get overlooked. PPE 64 Lab coats - evidence of use, adequate storage? Gloves HYGIENE/WELFARE Handwash facilities First aid kit /first aiders. Shower flushing In bio labs Howie style coats should be the standard. It is good practice to adopt this style for all laboratories. Coats should appear clean and there should be some formal regime of ensuring regular changing. There should be adequate hanging facilities inside laboratories [ not on corridors]. Coats should not be double hung on hooks or be hung on backs of chairs. Powdered latex gloves should not be in use, Nitrile are less allergenic and should be available for use. HWBs should be available in all labs near to entrance/exit. It should be clean and there should be a stock of soap and towels. In CL 2 and above taps should be elbow operated/or by PIR There should be a list of first aiders in the room or nearby. First aid kits should be fully stocked and a regular regime of checking contents in place. Eye wash bottles if present should be in date. Emergency eye and drench showers must be subject to regular weekly flushing - this should be recorded in the lab 65