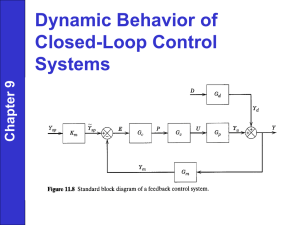
METHODS

Online Appendix for the following September 2 JACC article
TITLE: Dysregulation of Antioxidant Mechanisms Contributes to Increased Oxidative Stress in
Calcific Aortic Valvular Stenosis in Humans
AUTHORS: Jordan D. Miller, P
H
D, Yi Chu, P
H
D, Robert M. Brooks, BS, Wayne E.
Richenbacher, MD, Ricardo Pe~na-Silva, MD, Donald D. Heistad, MD
APPENDIX
ONLINE SUPPLEMENTARY METHODS
Patient Characteristics and General Histological Findings
Subjects in the control group were 57 ± 5 years old (range 21-79 years old), and patients undergoing valve replacement surgery for symptomatic aortic valve stenosis were 64 ± 3 years old (range 41-84 years old) (p = n.s.).
General Methods
Tissue . Healthy aortic valve tissue was acquired from donor hearts that were not suitable for transplantation (due to subclinical atherosclerosis, old age, no matching recipient, etc.) through the Iowa Donors Network and through the National Disease Research Interchange, Inc.
(Philadelphia, PA). Stenotic valve tissue was acquired from patients undergoing surgery to replace the aortic valve at the University of Iowa Hospital. Age and sex were the only
identifying information acquired from the surgical pathologist, and the University of Iowa
Human Subjects Committee deemed that informed consent from each patient was not required.
Histology . Valve tissue was frozen in OCT compound, and 10 micron sections were taken using a cryostat. Tissue mineralization and calcium deposition were visualized using Von
Kossa and Alizarin Red staining, respectively.
Detection of reactive oxygen species.
The spatial distribution of superoxide throughout the valve was determined using dihydroethidium fluorescence. In brief, sections of the valve were frozen in OCT compound and were cut in 10
M sections. These sections were then incubated in 0.002 mmol/L DHE and protected from light for 30 minutes at room temperature.
Separate slides were co-incubated in 0.002 mmol/L DHE and 200 Units/ml polyethylene glycol superoxide dismutase (PEG-SOD), and protected from light for 30 minutes at room temperature.
The sections were then imaged with a Bio Rad MRC-1024 laser scanning confocal microscope
(excitation 488 nm; emission 585 nm). The PEG-SOD-inhibitabe fraction of oxyethidium fluorescence for each region was determined using ImageJ software. Briefly, images were thresholded so that only the mean pixel intensity for punctate, positive staining was measured in the DHE-treated and DHE + PEG-SOD-treated images. The difference between these values was used as a measure of superoxide levels.
Calcified and non-calcified valve regions (almost always the base and tip of the stenotic valve, respectively) were carefully separated using sharp dissection, and lucigenin-enchanced chemiluminescence was used to measure superoxide levels. To control for spatial variations in superoxide levels that may normally occur in non-stenotic aortic valves, the healthy valve tissue also was divided into base and tip sections. The tissue was placed in 5
M lucigenin mixed in
phosphate-buffered saline, and fluorescence was read in a luminometer (Model FB12,
Zylux)(18).
The PEG-catalase-inhibitable fractions of dichlorofluorescein (DCF) fluorescence were used to estimate the levels and spatial distribution of hydrogen peroxide. Similar to the methods used for DHE fluorescence, sections of the valve were frozen in OCT compound and were cut in
10
M sections. These sections were then incubated in 0.005 mmol/L DCF and protected from light for 30 minutes at room temperature. Separate slides were co-incubated in 0.002 mmol/L
DCF and 2000 Units/ml polyethylene glycol-conjugated catalase (PEG-catalase), and protected from light for 30 minutes at room temperature. The sections were then imaged with a Bio Rad
MRC-1024 laser scanning confocal microscope (excitation 488 nm; emission 520 nm). The
PEG-catalase-inhibitable fraction of DCF fluorescence for each region was determined using
ImageJ software. Briefly, a threshold was set for images so that only the mean pixel intensity for fluorescent green positive staining was measured in the DCF-treated and DCF + PEG-catalasetreated images. The difference between these values was used as a measure of hydrogen peroxide levels.
Gene expression . Total RNA was isolated from calcific and non-calcific regions of stenotic valves and from the base and tip of “normal” valves using Trizol (Molecular Probes,
Inc.) coupled with Rneasy (Qiagen). Reverse transcription and real-time PCR were performed as described previously (20). Quantitative real-time RT-PCR was used to measure expression of mRNA for representatives of four groups of genes: 1) pro-calcific genes: tissue osteopontin and cbfa1; 2) pro-oxidant/NAD(P)H oxidase subunit genes: nox1, nox2, nox4, and p47 phox
; and 3) antioxidant genes: copper-zinc superoxide dimutase (CuZnSOD), manganese SOD (MnSOD),
extracellular superoxide dismutase (ecSOD), and catalase. All data are normalized to
-actin expression. All primers were purchased from Applied Biosystems.
Enzyme activity . NAD(P)H oxidase activity was assessed by measuring the tironinhibitable fraction of lucigenin-enhanced chemiluminescence (i.e., superoxide production) in response to 1, 10, or 100
M NADPH in homogenized valve tissue (0.5
g valve tissue/
l of reaction mixture). Total superoxide dismutase activity was measured using the absorbance of nitroblue tetrazolium as described previously (21,22).
We used homogenized aortic valve tissue and multiple pharmacological inhibitors to examine other potential enzymatic sources of superoxide (5
M lucigenin, 0.5
g/
l valve protein). Specifically, we examined the role of cyclooxygenases (10
M indomethacin), xanthine oxidase (100
M allopurinol), the nonspecific flavin inhibitor diphenyliodonium (DPI,
100
M), “uncoupled” nitric oxide synthase (100
M L-NAME), and apocynin (100
M apocynin). For this assay, changes are expressed as the change over the baseline value.
Immunohistochemistry . Immunostaining for alpha-smooth muscle actin (Sigma-Aldrich,
1:400), CD68/macrophages (AbD Serotec, 1:50), Msx1 (Sigma-Aldrich, 1:10), Msx2 (Sigma-
Aldrich, 1:10), and CBFA1 (R&D Systems, 1:20) was performed on individual slides using an appropriate Alexa Fluor 647 secondary antibody conjugate (Invitrogen) to minimize tissue autofluorescence. Slides were imaged using a Biorad 1024 confocal microscope equipped with a
Krypton-Argon laser using a 20x objective lens.
Statistics.
Group data are expressed as mean ± SE. Comparisons between normal and stenotic tissue were made using unpaired t-tests. Comparisons between groups were made using unpaired t-tests assuming unequal variances (Welch t-test). Bonferroni corrections were used to control for multiple comparisons. Significance was defined as
= 0.05.