supplementary information
advertisement

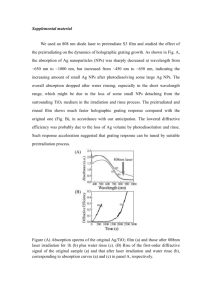
Supplementary information Ion irradiation synthesis of Ag–Au bimetallic nanospheroids in SiO2 glass substrate with tunable surface plasmon resonance frequency Xuan Meng1, Tamaki Shibayama2, a, Ruixuan Yu1, Shinya Takayanagi1, and Seiichi Watanabe2 1 Graduate School of Engineering, Hokkaido University, Sapporo, Hokkaido 060–8628, Japan 2 Center for Advanced Research of Energy and Materials, Faculty of Engineering, Hokkaido University, Sapporo, Hokkaido 060–8628, Japan S1. Ag nanoparticles long–term oxidation in vacuum condition. In general, the Ag nanoparticles are unstable and easily get oxidized1–3. Therefore, it was synthesized under the vacuum condition in our study. However, in the photoabsorption spectra measurement, it was exposed to ambient environment and it was therefore oxidized. Therefore, a layer of oxide Ag (usually Ag2O) formed on the nanoparticles surface, and the Ag–core Ag–oxide shell structures formed with a red shift of the characteristic surface plasmon resonance response peak possessed by pure Ag, which located at around 400 nm1, 2. After took the photoabsorption spectra and the consequent surface morphology examination by SEM, the sample was kept in vacuum box (<5 Pa). Eight months later, we took out the sample and took the photoabsorption spectra again, to compare with the optical response 8 months ago. The results are summarized in Fig. 4 (Fig. S–1 in supplementary information): the LSPR position has a very little shift towards the short wavelength for about 4.0 nm. Also, the intensity decreased a little, which can be interpreted by the long–term oxidation in the low vacuum condition with a low speed. aAuthor to whom correspondence should be addressed. E–mail: shiba@qe.eng.hokudai.ac.jp Fig. S–1 Photoabsorbance spectra of the sample (Ag(30nm)/SiO2) after 100 keV Ar ion irradiation at a fluence of 1.0×1017 cm−2 (pink dashed line); and (b) photoabsorbance spectra of the same sample taken 8 months later kept in vacuum condition (dark yellow solid line). S2. Surface plasmon resonance response of 20 nm Au films on SiO2 glass substrates under Ar ion irradiation. For the film thickness changed from 30 nm to 20 nm, a similar tendency of surface plasmon resonance response to irradiation fluence can be obtained. Optical absorption spectra were recorded for each specimen (Au(20 nm)/SiO2) after 100 keV Ar ion irradiation. Figure S–2a shows the photo absorption spectra for the specimens irradiated at fluences ranging from 2.5×1016 cm−2 to 1.2×1017 cm−2. For the specimen irradiated at a fluence of 2.5×1016 cm−2, a broad absorption peak was observed, while narrow absorption peaks were clearly observed for specimens irradiated at fluences exceeding 5.0×1016 cm−2. These absorption peaks corresponds to the localized surface plasmon resonance (SPR) peak possessed by Au nanoballs embedded in SiO2 4. This enhanced absorption is a characteristic of LSPR, and the peak positions are summarized in Figure S–2b. As the irradiation fluence increased, a shift of the LSPR peak positions toward the short wavelength was observed up to an irradiation fluence of 7.5 × 1017 cm−2, and then shifted towards the longer wavelength with further irradiation. Fig. S–2 (a) photoabsorbance spectra of the samples after 100 keV Ar ion irradiation at fluences ranging from 2.5×1016 cm−2 to 1.2×1017 cm−2; and (b) experimentally measured maximum absorption wavelength positions plotted against irradiation fluence. S3 Surface plasmon resonance response of 10 nm Au films on SiO2 glass substrates under Ar ion irradiation. For the film thickness changed to be 10 nm and the beam energy changed to be 250 keV, the ion induced blistering on the surface occurred, as verified by SEM and cross sectional TEM observations (Fig. S–3a and b). In the SEM image, we see the craters formed and the surface roughness increased. This was further verified by the TEM image, the white contrast just beneath the Au film showing the formation of Ar bubbles. The surface morphology is different from the 100 keV Ar ion irradiation of 30 nm Au film deposited on SiO2 to the same fluence (1.0 × 1017 cm−2). Furthermore, the photo absorption spectra were taken to evaluate the surface plasmon resonance response (Fig. S–4). Compared with the photo absorbance spectra for the 100 keV Ar ion irradiation of the pure SiO2 to a dose of 1.0 × 1017/cm2 (black dashed line), the absorbance band located around 550 nm corresponds to the surface plasmon resonance (SPR) peak possessed by Au nanoballs embedded in SiO2 fabricated by Ar ion irradiation of the 30 nm Au film on SiO2 substrate. However, the 250 keV irradiation resulted in a weak peak with suppressed intensity and shifted towards short wavelength. In general, the intensity and position of the LSPR peak are closely related to the size and volume fraction of the nanoballs embedded in the dielectrics. As the Au film thickness decreased from 30 nm to 10 nm, the average size of the Au nanoballs decreased and the shift of the LSPR peak towards the short wavelength was observed. Also, the volume fraction of the Au nanoballs in the dielectrics decreased, which resulted in the decreased absorption intensity. Fig. S–3: A typical SEM image of (a) 10 nm Au film on SiO2 irradiated by 250 keV Ar ions to a fluence of 1.0 × 1017 cm−2. (b) A typical bright field cross–sectional TEM image of 10 nm Au film on SiO2 irradiated by 250 keV Ar ions to a fluence of 1.0 × 1017 cm−2, with the carbon layer deposited on the upper part (indicated as C) to prevent the surface damage during cross sectional TEM sample preparation. Fig. S–4 Photo absorption spectra of the samples irradiated by Ar ions at 100 keV (blue solid line) and 250 keV (red dotted line) to a dose of 1.0×1017/cm2. Photo absorption spectra of the pure SiO2 irradiated with 100 keV Ar ions to a dose of 1.0×1017/cm2 was also obtained (black dashed line). References: 1M. Hillenkamp, G. D. Domenicantonio, O. Eugster and C. Félix, Nanotechnology 18, 015702 (2007). 2G. Valverde-Aguilar, J. A. García-Macedo, V. M. Rentería-Tapia, R. W. Gómez, M. Quintana-García, J. Nanopart. Res. 13, 4613–4622 (2011). 3 J. Hu, W. Cai, H. Zeng, C. Li and F. Sun, J. Phys.: Condens. Matter 18, 5415–5423 (2006). 4 X. Meng, T. Shibayama, R. Yu, S. Takayanagi, and S. Watanabe, J. Mater. Sci. 48, 920–928 (2013).