Methodology for a precise determination of the fusion enthapy by the
advertisement

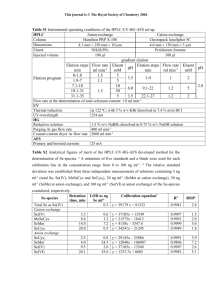
A new approach for the estimation of the melting enthalpy of metastable crystalline compounds using Differential Scanning Calorimetry: application to the two crystallographic forms of Etiracetam. Christelle Hermana,*, Tom Leyssensb, Valérie Vermylenc, Véronique Halloina, Benoît Hauta a Université Libre de Bruxelles, Transfers, Interfaces and Processes Department, Chemical Engineering Unit, 50 Avenue Franklin D-Roosevelt, CP 165/67, 1050 Bruxelles, Belgium b Université Catholique de Louvain, Institute of Condensed Matter and Nanosciences, 1 Place Louis Pasteur, 1348 Louvain-La-Neuve, Belgium c UCB Pharma, 60 Allée de la Recherche, 1070 Braine l’Alleud, Belgium * Corresponding author: Christelle Herman. E-mail: christelle.herman@ulb.ac.be. Tel: 0032 2 650 29 18. Fax: 0032 2 650 29 10. SUPPORTING INFORMATION TGA The thermogravimetric analysis was performed with a METTLER TOLEDO equipment. For the purpose of the experiment, approxiamtely 20 mg of Etiracetam was inserted into a 70 µl ceramic crucible. The temperature profile consisted in a dynamic segment from 303.15 K to 523.15 K. The evolution of the mass sample as a function of the temperature is presented in Fig. S1: Fig. S1a and S1b were obtained with a 5 K.min-1 and a 10 K.min-1 heating rates, respectively. Fig. S1 Mass sample as a function of the temperature: (a) 5 K.min -1 and (b) 10 K.min-1. Fig. S1 allows determining that a 0.5% mass loss occurs near to 419 K and 432 K for thermal analyses realized at 5 K.min-1 and 10 K.min-1, respectively. These temperatures are considered as the decomposition temperature and are presented by dotted lines in Fig. S1a and S1b. A 5% mass loss is observed near to 459 K and 468 K when using a heating rate of 5 K.min-1 and 10 K.min-1, respectively. The increase of the decomposition temperature obtained when increasing the heating rate is due to the thermal inertia. The decomposition temperature was at least 10 K above all of the final temperatures considered in the paper. In particular, the decomposition temperature is well above the melting temperature of both the form I and the form II, 385.44 K and 392.39 K, respectively. In both sub-figure of Fig. S1, the melting zone, in which both the form I and the form II melting occur, is highlighted in grey. Melting temperature The thermodynamic melting temperature of both forms was determined from measurements made under dynamic conditions following the methodology proposed by Höhne et al.. The onset temperature of the melting peak was measured at different heating rates and the value of the melting temperature was extrapolated to zero heating rate. Charsley et al. provide experimental confirmation that the extrapolation of the onset temperature at 1 a zero heating rate corresponds to the certified thermodynamic melting temperature. For this purpose, measurement crucibles were filled with 4 mg of form I or form II crystals. The temperature profile of the thermal analyses consisted in a sole dynamic segment from an initial to a final temperature without an isothermal segment at the initial temperature nor at the final temperature. The temperature domain extended from 303.15 K to 403.15 K. Various heating rates were used: 1 K.min -1, 2 K.min-1, 5 K.min-1, 10 K.min-1, 20 K.min-1 and 40 K.min-1. All experiments were performed with the Tau Lag option disabled. This option otherwise automatically corrects for the inertness of the system towards heat transfer when experimental heating rates used are different to the rate used for the initial temperature calibration. The thermograms, obtained in these conditions, for the form I and the form II are presented in Fig. S2. Fig. S2 Thermograms at different heating rates of (a) the form II (113°C = 386.15K and 129°C = 402.15K) and (b) the form I (111°C = 384.15K and 129°C = 402.15K). The melting temperature of each form was obtained by a three steps method. In a first step, the onset of the melting peak on each of the thermograms presented in Fig. S2a and Fig. S2b was determined. In a second step, a linear relationship between the onset temperature and the applied heating rate was determined by the least-square fitting method (Fig. S3a and Fig. S3b). In a third step, the thermodynamic melting temperature was obtained extrapolating this linear relation at a zero heating rate (Fig. S3a and Fig. S3b). The thermodynamic melting temperature of the form II is shown to be 392.39 K ± 0.04 K (Fig. S3a). The thermodynamic melting temperature of the form I is shown to be 385.44 K ± 0.04 K (Fig. S3b). The uncertainty 2 on these temperatures was calculated determining the 95% confidence interval on the constant of the linear regression. This leads to relative uncertainties of approximately 0.01%. Fig. S3 Linear regression between the melting peak onset temperature and the heating rate for () the form II and (b) the form I. Höhne G.W.H., Cammenga H.K., Eysel W., Gmelin E., Hemminger W. The temperature calibration of Scanning Calorimeters. Thermochimica Acta. 1990;160:1-12 Charsley E.L., Laye P.G., Palakollu V., Rooney J.J, Joseph B. DSC studies on organic melting point temperature standards. Thermochimica Acta. 2006;446 :29-32 Specific heat capacities The specific heat capacities of both crystallographic forms, as well as the specific heat capacity of the liquid phase, were determined using the so called “sapphire method”. This method is advisable when high accuracy is required. The method consists in determining the specific heat capacity of a studied crystalline compound by comparing the baseline displacement between the sample thermogram and the thermogram of a compound of known specific heat capacity (sapphire). Both thermograms have to be corrected by a blank thermogram. The three thermograms (sample, sapphire and blank) have to be obtained using the same temperature profile. The imposed temperature profiles consisted in a 5 minutes isothermal segment at an initial temperature followed by a dynamic segment from the initial temperature to a final temperature and ended by a 5 minutes isothermal segment at the final temperature. The heating rate used was 15 K.min-1. For the solid phases, the initial temperature was 298.15 K and the final temperature was 373.15 K. For the liquid phase, the initial temperature was 403.15 K and the final temperature was 428.15 K. The high heating rate used (15 K.min-1) allowed avoiding solid-solid polyformic transitions from the metastable form I to the stable form II within the temperature range of interest. At the final temperature chosen, the decomposition has not yet occurred. These temperature profiles were applied to an empty crucible. The resulting thermograms were used as a blank. The crucible was then filled with 20 mg of sapphire and submitted to the same temperature profiles in order to obtain the sapphire thermograms. A new crucible, whose mass corresponded closely to the mass of the previously used crucible, was filled with 20 mg of form I or form II crystals. Once more, the same temperature profiles were applied to obtain the thermograms of the sample. In order to evaluate the robustness of the method, various independent sets of experiments were performed for each phase: 6 for the form I crystals, 4 for the form II crystals and 4 for the liquid phase. In Fig. S4, experimentally determined specific heat capacities are presented as a function of the temperature for the three phases (form I crystals, form II crystals and liquid phase). The form I melting temperature, the form II melting temperature and the decomposition temperature are also presented in Fig. S4. The zone highlighted in grey is the zone in which Etiracetam decomposes. 3 Fig. S4 Specific heat capacities of the Etiracetam compound as a function of the temperature. form I crystals: 6 sets of experiments between 298.15 K and 373.15 K. form II crystals: 4 sets of experiments between 298.15 K and 373.15 K. Liquid phase: 4 sets of experiments between 403.15 K and 428.15 K. form I melting temperature: 385.44 K. form II melting temperature: 392.39 K. Decomposition temperature: near 419 K (5 K.min-1) - 432 K (10 K.min-1). For each phase, a least-square linear regression was performed on the average values of the experimental results to yield a linear relation between the specific heat capacity and the temperature (cp=a+bT). These linear relations are presented in Table S1. The low R2 encountered for the liquid phase is due to the limited temperature range investigated (from 403.15 K to 428.15 K). In Table S1, the values of the specific heat capacities of the solid (form I and form II) and the liquid phases are presented at three temperatures: 303.65 K, the form I melting temperature (Tm,I) and the form II melting temperature (Tm,II). As expected the specific heat capacity of the melting temperature varies significantly comparing solid to liquid phases. Furthermore, the specific heat capacity of the solid phase is about 3 times more temperature dependent than the heat capacity of the liquid phase. For each phase and for each experimental temperature in the temperature range investigated, the 95% confidence interval for the determination of the specific heat capacity was determined. This leads to relative uncertainties of approximately 2% - 5%, which are of the expected precision for experimental DSC measurements of the specific heat capacity. Table S1 Linear relation between the specific heat capacity and the temperature and specific heat capacities at three temperatures for the solid phases (form I and form II) and the liquid phase of Etiracetam. (*: extrapolated values). T/K cp / J.g-1.K-1 cp (303.65 K) / J.g-1.K-1 cp (Tm,I) / J.g-1.K-1 cp (Tm,II) / J.g-1.K-1 Form I -0.360 + 0.0059 T [298.15 – 373.15] 1.431 ± 0.04 1.913* crystals R2 = 0.80 Form II -0.111 + 0.005 T [298.15 – 373.15] 1.407 ± 0.04 1.850* crystals R2 = 0.79 Liquid 1.589 + 0.0017 T [403.15 – 428.15] 2.244* 2.256* phase R2 = 0.08 de Buhr J., Riesen R., Widmann J., Jörimann U., Informations pour les utilisateurs des systèmes d’analyses thermiques METTLER TOLEDO: mesure de la chaleur spécifique. User Com 7. 1998 Determination of the form II enthalpy The following Tables presents, for each heating rate, the experimental conditions of initial and final temperatures used for the determination of the form II melting enthalpy: Table S2 for 1 K.min-1, Table S3 for 2 K.min-1, Table S4 for 5 K.min-1 and Table S5 for 10 K.min-1. In each Table, X represents the experimental conditions whose results are presented in the article; X represents the experimental conditions whose results are presented in the Supporting Information; x represents the experimental conditions whose results are not presented in the article either in the Supporting Information; the experimental conditions highlighted in grey are the ones selected for the extraction of the melting enthalpy 4 Tf / K Ti / K 387.15 387.65 388.15 388.65 389.15 389.65 390.15 390.65 391.15 X X X X X X X X X Tf / K Ti / K 387.15 387.65 388.15 388.65 389.15 389.65 390.15 390.65 391.15 x x x x x x x x X X X X Table S3 Experimental initial and final temperatures for 2 K.min-1 396.15 396.65 397.15 397.65 398.15 X X X X X X X X X Tf / K Ti / K 387.15 387.65 388.15 388.65 389.15 389.65 390.15 390.65 391.15 Table S2 Experimental initial and final temperatures for 1 K.min-1 394.65 395.15 395.65 396.15 396.65 x x x x x x x x X X X X Table S4 Experimental initial and final temperatures for 5 K.min-1 398.65 399.15 399.65 400.15 400.65 X X X X X X X X X x x x x x x x x X X X X 397.15 x x x x x x x x X 398.65 x x x x x x x x X 401.15 x x x x x x x x X Table S5 Experimental initial and final temperatures for 10 K.min-1 Tf / K 402.15 402.65 Ti / K 387.15 X X 387.65 X X 388.15 X X 388.65 X X 389.15 X X 389.65 X X 390.15 X X 390.65 X X 391.15 X X Fig. S5 and Fig. S6 present the experimental results extracted from the thermograms obtained when heating the form II at 2 K.min-1 and 10 K.min-1, respectively. As for the Fig. 8 and 9 presented in the article, in these figures, the form II melting enthalpy obtained by the method presented in this work (Met.) is compared with the results obtained by the conventional method using the ‘integral horizontal’ (Int. Hor.) and the ‘integral tangential’ (Int. Tang.) virtual baselines. The influence of the choice of the initial temperature is presented in Fig. S5a and Fig. S6a for the lowest final temperatures: 396.15 K for 2 K.min -1 and 402.65 K for 10 K.min-1, respectively. The influence of the choice of the final temperature is presented in Fig. S5b and Fig. S6b for the largest initial temperature (391.15 K) for 2 K.min-1 and 10 K.min-1, respectively. 5 Fig. S5 Melting enthalpy of the form II extracted from the thermogram obtained at 2 K.min-1 with the conventional methods ‘Int. Hor.’ and ‘Int. Tang.’ and with the method presented in this work (‘Met.’). Influence of the choice of (a) the initial temperature for a final temperature = 396.65 K and (b) the final temperature for an initial temperature = 391.15 K. Fig. S6 Melting enthalpy of the form II extracted from the thermogram obtained at 10 K.min-1 with the conventional methods ‘Int. Hor.’ and ‘Int. Tang.’ and with the method presented in this work (‘Met.’). Influence of the choice of (a) the initial temperature for a final temperature = 402.65 K and (b) the final temperature for an initial temperature = 391.15 K. Estimation of the form I enthalpy The following Tables present, for each heating rate, the experimental conditions of initial and final temperatures used for the determination of the form I melting enthalpy: Table S6 for 2 K.min-1, Table S7 for 5 K.min-1 and Table S8 for 10 K.min-1. In each Table, X represents the experimental conditions whose results are presented in the article and the experimental conditions highlighted in grey are the ones selected for the extraction of the melting enthalpy. 6 Table S6 Experimental initial and final temperatures for 2 K.min-1 Tf / K 396.15 396.65 397.15 Ti / K 367.15 X X X 367.65 X X X 368.15 X X X 368.65 X X X 369.15 X X X Table S7 Experimental initial and final temperatures for 5 K.min-1 Tf / K 399.15 399.65 400.15 Ti / K 373.15 X X X 373.65 X X X 374.15 X X X 374.65 X X X 375.15 X X X 375.65 X X X 376.15 X X X Table S8 Experimental initial and final temperatures for 10 K.min-1 Tf / K 401.65 402.15 402.65 Ti / K 375.15 X X X 375.65 X X X 376.15 X X X 376.65 X X X 377.15 X X X 377.65 X X X 378.15 X X X 378.65 X X X 379.15 X X X 379.65 X X X 380.15 X X X Fig. S7 presents the experimental form I melting enthalpy extracted from the thermograms obtained when heating the form I at (a) 2 K.min-1, (b) 5 K.min-1 and (c) 10 K.min-1. The form I melting enthalpy was estimated by the method presented in this work. Figure S4 consists in a zoom of each sub-figure of Fig. 11 given in the article. 7 Fig. S7 Melting enthalpy of the form I extracted from the thermogram obtained at (a) 2 K.min -1, (b) 5 K.min-1 and (c) 10 K.min-1 with the method presented in this work. Influence of the choice of the initial temperature for 3 final temperatures. Zoom of each sub-fig. of Fig. 11. 8