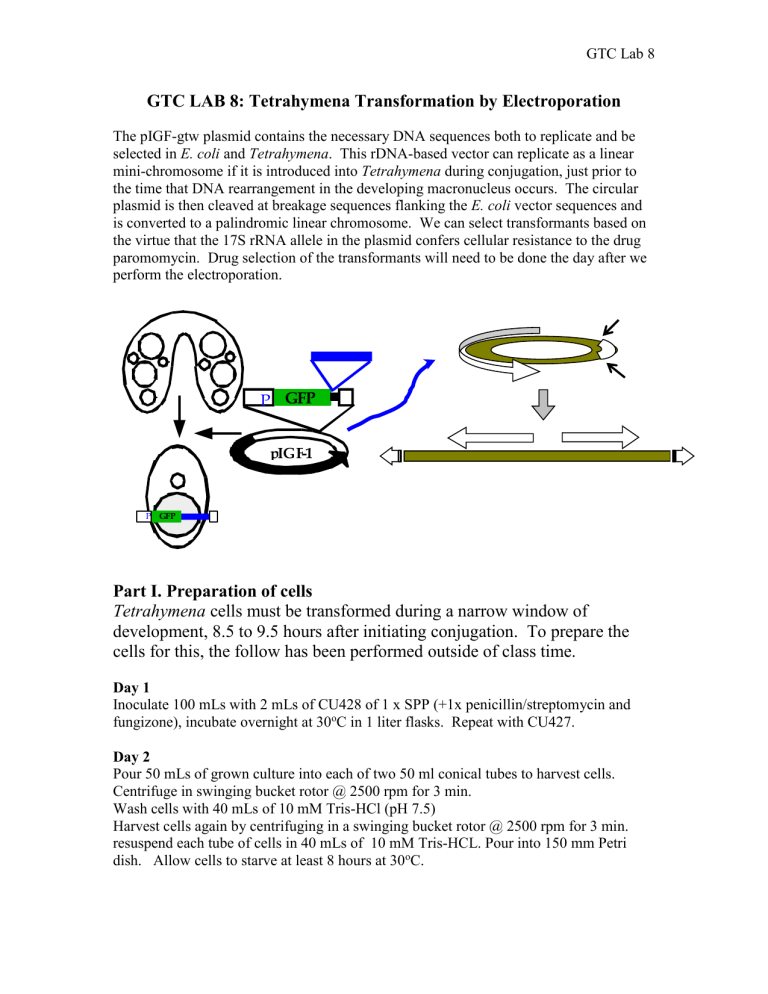
GTC LAB 8: Tetrahymena Transformation by Electroporation
GTC Lab 8
GTC LAB 8: Tetrahymena Transformation by Electroporation
The pIGF-gtw plasmid contains the necessary DNA sequences both to replicate and be selected in E. coli and Tetrahymena . This rDNA-based vector can replicate as a linear mini-chromosome if it is introduced into Tetrahymena during conjugation, just prior to the time that DNA rearrangement in the developing macronucleus occurs. The circular plasmid is then cleaved at breakage sequences flanking the E. coli vector sequences and is converted to a palindromic linear chromosome. We can select transformants based on the virtue that the 17S rRNA allele in the plasmid confers cellular resistance to the drug paromomycin. Drug selection of the transformants will need to be done the day after we perform the electroporation.
P G F P
P p I
G
G
F
F -
P
1
Part I. Preparation of cells
Tetrahymena cells must be transformed during a narrow window of development, 8.5 to 9.5 hours after initiating conjugation. To prepare the cells for this, the follow has been performed outside of class time.
Day 1
Inoculate 100 mLs with 2 mLs of CU428 of 1 x SPP (+1x penicillin/streptomycin and fungizone), incubate overnight at 30 o
C in 1 liter flasks. Repeat with CU427.
Day 2
Pour 50 mLs of grown culture into each of two 50 ml conical tubes to harvest cells.
Centrifuge in swinging bucket rotor @ 2500 rpm for 3 min.
Wash cells with 40 mLs of 10 mM Tris-HCl (pH 7.5)
Harvest cells again by centrifuging in a swinging bucket rotor @ 2500 rpm for 3 min. resuspend each tube of cells in 40 mLs of 10 mM Tris-HCL. Pour into 150 mm Petri dish. Allow cells to starve at least 8 hours at 30 o
C.
GTC Lab 8
Approximately 9 hours prior to electroporation (Part III below), mix equal numbers of pre-starved Tetrahymena of complementary mating types. Allow cells to pair for conjugation at 30 o C, no shaking.
Day 3 – Electroporation
Part II. Preparation of DNA
1. Using the “boiling lysis” method of isolating plasmids (GTC LAB 6), isolate plasmid
from 5 mLs of E. coli containing the correct clone. This can be done in two
microcentrifuge tubes by first centrifuging 1.4 mL of cells in each, discarding the
supernatant, then adding another 1mL of cell culture and repeating.
2. Resuspend the final pellets in 100 µL of TE buffer, each. Add 1 µL of RNase A
(10mg/mL stock) and incubate at 37ºC for 15 minutes.
3. Extract the preparations once with 100 µL phenol:chloroform:isoamyl alcohol
(25:24:1).
4. Add 10 µL of 3M NaOAc and 300 µL of isopropanol. Let mixtures chill on ice for 15
minutes.
5. Collect the DNA precipitates by centrifuging at maximum speed for 15 minutes.
6. Dry the pellets and resuspend each in 25 µL of sterile 10 mM HEPES buffer.
Part III. Electroporation
1. Transfer 40 mLs of conjugating cells to 50 ml sterile conical centrifuge tubes (using
25- mL pipettes) and spin down for 3 minutes at 2500 RPM in a swinging bucket rotor if possible. Decant supernatant quickly.
Note: It is important to decant medium as soon as centrifuge stops as the
Tetrahymena will try to rapidly swim out of the pellet.
*This volume of cells will be enough for two transformations
2. After pouring off supernatant and gently resuspending in the remaining liquid (by
taping tube with finger), add 40 mLs of 10 mM HEPES buffer. Incubate at room
temperature for 5 minutes.
4. Harvest the 40 mLs of cells again by centrifuging @ 2500 rpm for 3 min.
Resuspend cells in 400 µl HEPES buffer and proceed with electroporation.
5. Turn on the electroporater (BTX) and set up with capacitance=275µF ,
resistance=25Ω, Voltage = 250V. Double-check all settings.
GTC Lab 8
Procedure: For EACH sample of mating cells to be electroporated:
1. Combine the transforming DNA from both tubes into one sterile tube.
2. Using a P-1000, add 200 µl of the mating cells to the DNA in the microcentrifuge tube.
3. Mix gently by pipeting up and down.
4. Gently pipet the DNA+cell mixture from the microcentrifuge tube into a 4mm gap cuvette.
5. Put the cuvette in the electroporation chamber and push the pulse button. Note time.
Record peak voltage and duration (msec.)
6. Remove the cuvette from the chamber. Press the reset button.
7. Wait one minute after electroporation, then wash the cells out of the cuvette by adding
0.5 mL of growth medium and pipeting cells with a Pasteur pipette (or P-200 pipet) into a tube containing 5 mL of growth medium (1XSPP + 1X pen/strep + 1X fungizone).
Repeat the above for all samples . Flush the cuvette with water and 70% ETOH to sterilize.
Plating of Cells:
1. Make 30 mLs of a 1:10 dilution of electroporated cells by mixing 27 mLs of SPP (+ 1x pen/strep, 1x fungizone) and 3 mLs of electroporated cells.
2. Pour cell mixture into a media tray.
3. Plate each sample into 3 separate 96 well plates, aliquoting 100
l/well using multichannel pipettors.
Incubate plates at 30ºC for 16 hours before adding drug to select transformants.
Drug will be added to your transformation plates the next day. Make the necessary arrangements with your lab instructor.
Next day (Selecting Tetrahymena electroporation transformants)
1. Take 30 mLs of media containing 200 µg/mL paromomycin from the prepared stock.
2. Pour media into a media reservoir.
3. Aliquot 100
l/well to each of the three 96 well plates containing cells. Use a
multichannel pipettor.
4. Incubate at 30ºC for at least 3 days before checking for transformants.
GTC Lab 8
Part IV: Maintaining transformants
Overview of steps
1.
Score the number of total transformants
2.
Select 6-12 transformants from each shot for future experimentation
For each construct that was electroporated into Tetrahymena, identify wells containing paromomycin resistant transformants. Select a reasonable number (six to twelve, if you have this many) to propagate for further experiments.
1. Pipet 1.5 mLs of 1x SPP medium + 100 g/mL paromomycin + 1 x pen/strep into each
well of a 24-well plate that will be used to grow transformants.
2. Select the drug resistant transformants for each construct that you will transfer.
3. For each selected transformant, aliquot 30
l of each to the new well with media in the
24-well plate. Label each well .
4. Incubate plates in Tupperware boxes at 30 o
C for 1-2 days to allow transformant
populations to grow.