B6_UK-1
advertisement

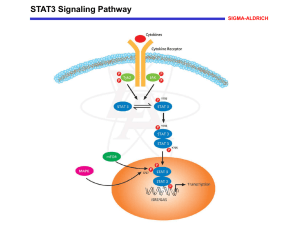
B6 Switching towards Termination – Modulation of the Dynamic Behaviour of the SMAD Signaling Cascade Projectleaders: Experimental: PD Dr. U. Klingmüller, DKFZ, Heidelberg, +49 6221 42 4481, u.klingmueller@dkfz-heidelberg Modeling: Prof. J. Timmer, Center for Data Analysis and Modeling, University of Freiburg, +49 761 203 5829, jeti@fdm.uni-freiburg.de Summary: Based on time-resolved quantitative data for the activation of the SMAD signaling cascade in primary hepatocytes (Klingmüller et al., IEE Proc Systems Biology) we established a data-based mathematical model indicating the importance of negative feed-back loops for determining the dynamic behavior of the signaling cascade. To extand this model we will examine the activation of the transforming growth factor (TGF)beta -activated kinase (TAK)1 and the Nemo-like kinase (NLK). To understand mechanisms promoting the switch towards termination of hepatocyte regeneration we will monitor cross-talk with other signaling cascades that could influence signal ingtegration by the TGFbeta stimulated signaling. Since recent evidence suggests that serine phosphorylation of STAT3 contributes to TGFbeta-mediated developmental processes, potentially including termination of hepatocyte regeneration, we will compare the induction of target genes in response to TGFbeta stimulation in normal versus hepatocytes obtained from S727A STAT3 knock-in mice (collaboration with B1). Furthermore, we will examine the induction of the phosphoinositide 5 phosphatase SHIP-1 that is a target gene of SMAD2/3. Since SHIP-1 is an important regulator of PI3 kinase signaling that is activated by the Met receptor during the proliferative phase, we will focus in collaboration with B5 on the suppressive effects of SMAD signaling on c-met receptor signaling.The stoichimoetry of pathway components and cell state specific alterations will be monitored by quantitative proteomics. Target gene induction will be examined initially by microarray analysis and later on by qRT-PCR. To address spatial effects, fluoresecently labeled SMAD and TAK1 will be expressed at endogenous levels by applying the Tet-inducible system developed in B5. The dynamics of nuclearcytoplasmic cycling of SMAD will be quantitatively monitored by live cell imaging and the kinetics of the TAK1 and STAT3 interaction will be analyzed by fluorescence resonance energy transfer. The aquired data will be used to establish a spatiotemporal model. The comprehensive model of SMAD signaling will be employed to identify mechanisms promoting the coordinated induction of the regeneration of hepatocytes. Work Plan: Year 1: (a) We will monitor the time course of TAK-1 activation in response to TGFbeta stimulation and compare it to the acctivation of SMAD2 and 3. Furthermore the impact of TGFbeta stimulation on the extent and duration of STAT 3 serine phosphorylation will be analyzed by quantitative immunoblotting. (b) To monitor the transition between the priming and the proliferative phase assays to quantify the induction of DNA synthesis in primary hepatocytes will be established at a single cell level by Bromodeoxyuridine (BrdU) incorporation and in the cell population by measuring the incorporation of 3H-Thymidine. (c) To determine the stochiometry of the pathway components and determine absolute numbers we we will purify in addition to SMAD2 and 3, TGFbeta type I and II receptor, TAK-1 and determine phosphorylated and unphosphorylated peptides suitable for quantitative analysis (based on the experience in the sugar grant and collaboration with A3). Year 2 (a) Time-resolved quantitative data for activation of TAK-1 will be used to extend our model for the TGFbeta stimulated activation of the SMAD signaling cascade. Furthermore TGFbeta induced target genes will be quantified by Realtime PCR and used to extend the mathematical model. (b) Quantitative measurements by massspectrometry for selected time points and comparison to quantitative immunoblotting. (c) Establishment of GFP-tagged SMAD2 and 3 and of fluorescently labeled TAK1 suitable for FRET analysis with STAT3. Year 3 (a) Incorporation of the autocrine loop and other modes of cross-talk into our mathematical model. Predicting events required for the transition from priming phase towards proliferation and experimental validation. (b) Experimental validation of the importance of serine phosphorylatin of STAT3 on TGFbeta mediated responses. The time course of TGFbeta stimulated signaling pathways and target gene activation in primary hepaotcytes from S727A STAT3 knock-in mice. (c) Time-resolved quantitative proteomics and quality control by quantitative immunoblotting. (d) Spatio-temporal modeling of nuclear-cytoplasmic cycling of SMAD2 and 3 and of the cross-talk with the JAK1-STAT3 signaling cascade. Milestones: Extending our SMAD signaling cascade model by incorporating the dynamics of TAK-1 and NLK-1 activation Establishing reliable assays to monitor the induction of DNA synthesis in primary hepatocytes Identifiying the cross-modulatory effects on STAT3 signaling Determining the dynamics of SHIP-1 induction Identification of the suppressive effects on c-met receptor signaling Establishing a spatio-temporal model of SMAD2 and 3 nucelar-cytoplasmic cycling and cross-talk with the JAK1-SAT3 signaling cascade. Budget Klingmüller Personal: One graduate student (BATIIa/2) with knowledge in molecular biology and cell biology as well as an interest in live cell imaging Consumables: Preparation, cultivation and stimulation of hepatocytes (purchasing BL6 mice, collagenase, collagene treated plates, FCS, Williams medium, TGFbeta and TNFalpha) 73.780 10.000 Quantitative immunoblotting (primary antibodies for TGFbeta type I and II, SMAD2, 3, 4, 7, SnoN, TAK1, NKL-1, STAT3, phospho-specirif antibodies and secondary antibodies, nitrocellulose/PVDF membrane, BSA, recombinant proteins as calibroators and antibodies against actin, HSC70, PDI, calnexin, clathrine as normalizer) Mass spectrometry (recombinant proteins for the identification of suitable peptides and isotope labeled or mutated as standards, isotope labeled peptides (AQUA technology from Sigma) Generation of fluorescent labeld SMAD2 and 3 and TAK1 and live cell imaging (restriction enzymes, chambers for live cell imaging), Target gene activation (Affymetrix microarrays, primers and polymerase for qRT-PCR) 20.000 10.000 20.000 60.000 Budget Timmer Personal: One graduate student (BATII/a/2) for mathematical modeling 85.650 Budget Travel: 4 trips to Freiburg per year per person for the experimental group, 4 trips to Heidelberg for the modeling group, participation in 1 European conference in the first two years and 1 international conference in the third year. 10.000 Gemeinkosten Is doch jetzt geklärt fürs DKFZ, wie viel darf da angesetzt werden ????? References: Heinrich, A. C., R. Pelanda, U. Klingmüller. A Mouse Model for Visualization and Targeted Mutations in the Erythroid Lineage. Blood. (2004) 104(3):659-66. Klingmüller, U., A. Bauer, S. Bohl, P. J. Nickel, K. Breitkopf, S. Dooley, S. Zellmer, C. Kern, I. Merfort, T. Sparna, J. Donauer, G. Walz, M. Geyerr, C. Kreutz, M. Hermes, F. Götschel, A. Hecht, D. Walter, L. Egger, K. Neubert, C. Borner, M. Brulport, W. Schormann, C. Sauer, F. Baumann, R. Preiss, S. MacNelly, P. Godoy, E. Wiercinska, L. Ciuclan, P. Illes, K. Zeilinger, M. Heinrich, U. M. Zanger, M. Reuss, A. Bader, R. Gebhardt, T. Maiwald, J. Timmer, F. von Weizsäcker, J. G. Hengstler Primary mouse hepatocytes for systems biology approaches: a standardized in vitro system for modeling of signal transduction pathways. IEE Proc Systems Biology in press. Schilling, M., T. Maiwald, S. Bohl, M. Kollmann, C. Kreutz, J. Timmer, U. Klingmüller. Computational Processing and Error Reduction Strategies for Standardized Quantitative Data in Biological Networks. FEBS Journal, 272, 6400-6411. Schilling, M., T. Maiwald, S. Bohl, M. Kollmann, C. Kreutz, J. Timmer, U. Klingmüller. Quantitative Data Generation for Systems Biology – The Impact of Randomisation, Calibrators and Normalisers. In press in IEE Proc Systems Biology. Swameye, I., T. G. Müller, J. Timmer, O. Sandra, U. Klingmüller. Identification of nucleocytoplasmic cycling as a remote sensor in cellular signaling by data-based dynamic modeling. PNAS (2003) 100:1028-33.