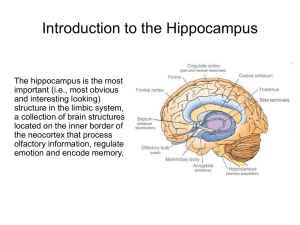
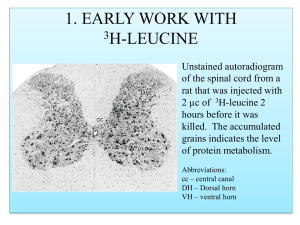
Finally, the peak firing rate within any one place field of a single cell
advertisement

MODELS, STRUCTURE, FUNCTION: THE TRANSFORMATION OF CORTICAL SIGNALS IN THE DENTATE GYRUS László Acsády1 and Szabolcs Káli1,2 1 Institute of Experimental Medicine, Hungarian Academy of Sciences, PO Box 67, 1450 Budapest, Hungary, 2 HAS-PPCU-SU Neurobionics Research Group, Budapest, Hungary Corresponding author: László Acsády Institute of Experimental Medicine, Hungarian Academy of Sciences, PO Box 67, 1450 Budapest, Hungary, email: acsady@koki.hu; tel: 36-1-210-9413; fax: 36-1-210-9412 2 Abstract Our central question is why the hippocampal CA3 region is the only cortical area capable of forming interference-free representations of complex environmental events (episodes), given that apparently all cortical regions have recurrent excitatory circuits with modifiable synapses, the basic substrate for autoassociative memory networks. We review evidence for the radical (but classic) view that a unique transformation of incoming cortical signals by the dentate gyrus and the subsequent faithful transfer of the resulting code by the mossy fibers are absolutely critical for the appropriate association of memory items by CA3 and, in general, for hippocampal function. In particular, at the gate of the hippocampal formation, the dentate gyrus possesses a set of unusual properties which selectively evolved for the task of code transformation between cortical afferents and the hippocampus. These evolutionarily-conserved anatomical features enable the dentate gyrus to translate the noisy signal of the upstream cortical areas into the sparse and specific code of hippocampal formation, which is indispensable for the efficient storage and recall of multiple, multidimensional memory items. To achieve this goal the mossy fiber pathway maximally utilizes the opportunity to differentially regulate its postsynaptic partners. Selective innervation of CA3 pyramidal cells and interneurons by distinct terminal types creates a favorable condition to differentially regulate the short-term and longterm plasticity and the motility of various mossy terminal types. The utility of this highly dynamic system appears to be the frequency-dependent fine-tuning of the excitation evoked by the large “detonator” terminals and the inhibition activated by the small terminals. This will determine exactly which CA3 cell 3 population is active and induce permanent modification in the autoassociational network of the CA3 region. Introduction The hippocampus, a peculiar cortical structure, has long been known to be involved in higher order cognitive functions, most notably, memory formation and spatial navigation (Scoville & Milner, 1957; O'Keefe & Dostrovsky, 1971). Not all kinds of memory depend on the hippocampus. The learning of arbitrary associations of complex, multimodal items during a single exposure (i.e., episodic memory) is irreversibly compromised following hippocampal lesions, but the acquisition of simple associative pairings (e.g., classical conditioning) and motor learning remain intact (Squire, 1992). Hippocampal-dependent memory traces can be used flexibly, i.e., they can be activated in a context different from the one where they were learned. Clinical studies in humans, as well as a large number of behavioral and physiological experiments in other mammalian species (mainly rodents) have also implicated the hippocampus in the formation and flexible use of world-centered (allocentric) spatial representations (O'Keefe & Nadel, 1978). It has been argued repeatedly that these two domains share several important characteristics, and may have fairly similar computational requirements. In particular, both episodic memory and spatial navigation may require the fast storage and subsequent recall of specific conjunctions of environmental stimuli. It has been suggested that the bulk of neocortex, which is assumed to be involved in continuously creating and refining an internal representation of the general structure of the observed world, may not be well-suited for the rapid, interference-free acquisition of such specific memory traces(McClelland et al., 1995). On the other hand, the hippocampus might be 4 optimized for exactly this operation, and could thus complement the generic learning capabilities of the neocortex. However, the hippocampus constitutes only a tiny fraction of the cortical areas, and it has a relatively simple structure. It receives input from and sends information back to multimodal associational cortical areas. The question is why neocortex needs the hippocampal loop to implement rapid learning of arbitrary complex associations? Why is it that other cortical areas with multiple cellular layers cannot do the same job? What is so special about hippocampus that enables it to establish the most complex memory traces? In short, what is the “trick” of the hippocampus? For a long time, the central role for memory formation was assigned to the hippocampal CA3 region (Marr, 1971; McNaughton & Morris, 1987; Rolls, 1989). This hippocampal subfield was favored by most computational neuroscientists and electrophysiologists since its principal cells, the CA3 pyramidal cells, form a socalled “autoassociative memory network” with their abundant, local recurrent collaterals (Li et al., 1994). In computational models these types of network were found to be optimal for the efficient storage of a large number of memory items and for reactivation of complete memory traces if only part of the trace was provided, which are key components of episodic memory. Moreover, the synapses among CA3 pyramidal cells, as well as those between pyramidal cells in CA3 and their primary downstream target CA1, are subject to associative and cooperative long-term potentiation (LTP), a possible molecular mechanism underlying memory formation (Debanne et al., 1998). Interestingly, however, many features of CA3 pyramidal cells are shared by pyramidal cells of other cortical areas. Neocortical pyramidal cells have just as profuse recurrent local collaterals and their synapses are subject to longterm plastic changes. CA3 and layer II-III cells also have many intrinsic 5 electrophysiological characteristics in common. Thus, autoassociative networks with plastic synapses are abundant in the cortex. Therefore, it is not immediately obvious why the formation of complex memory traces is restricted to the hippocampal formation and cannot be performed by other multimodal associational cortical areas. Two features of the CA3 area, however, clearly distinguish it from other cortical regions. CA3 pyramidal cells receive a prominent excitatory input to their proximal apical dendrites (Ramon y Cajal, 1911; Claiborne et al., 1986), which enables an unusually strong spike coupling between an upstream region – the dentate gyrus – and CA3 (Henze et al., 2002). The faithfulness of this synaptic transmission is unparalleled in excitatory cortical circuits. The second feature (shared by the principal cells of the other hippocampal subregions) is the way in which the firing pattern of pyramidal cell codes the environmentally-relevant stimuli. Most pyramidal cells in other cortical regions are characterized by higher spontaneous firing rates, and a lower rate of modulation by the appropriate stimuli. In sharp contrast, background activity in hippocampal principal cells, and granule cells in particular, is very low (Muller et al., 1987; Barnes et al., 1990; Quirk et al., 1992; Jung & McNaughton, 1993). However, when hippocampal principal cells participate in information transfer (e.g. place cells), their activity increases enormously. In computational terms, the hippocampus uses a sparse code, whereas coding in other cortical areas is denser. Apparently, the hippocampus and the other cortical regions “speak” different neuronal languages. Since the environmentally-specific information reaches the hippocampus via other cortical areas, the immediate consequence of the difference in coding strategies is that densely-encoded cortical information reaching the hippocampus needs to be translated into a sparser hippocampal code. In other words, an interface is needed between the hippocampus and neocortex. The main task of this interface would be the translation of the 6 neocortical code into a hippocampal one, and to transfer the new code as efficiently as possible to the next station of information processing. According to the classic view, this interface is the dentate gyrus (DG), the first step of the trisynaptic hippocampal loop. We argue that the dentate gyrus is the “odd-man-out” among cortical regions. In particular, at the gate of the hippocampal formation, the dentate gyrus possesses a set of unusual properties that selectively evolved for the task of code transformation between cortical afferents and the hippocampus. These evolutionarily-conserved anatomical features enable the dentate gyrus to translate the noisy signal of the upstream cortical areas to the sparse and specific code of hippocampal formation, which is indispensable for the formation of multiple, multidimensional memory items. Computational requirements for the formation of episodic memories There is a sort of general consensus about how the hippocampus could contribute to cortical memory functions (Alvarez & Squire, 1994; Treves & Rolls, 1994; McClelland et al., 1995; Kali & Dayan, 2004; Rolls & Kesner, 2006). First, the hippocampus is assumed to be capable of rapidly creating and storing a memory trace which is distinct from all existing traces. The memory trace is associated with a snapshot of activity in medial temporal neocortex (particularly entorhinal cortex), which, in turn, is thought to represent a compressed version of activity in the rest of neocortex. Second, the hippocampus is thought to be capable of retrieving particular stored traces if the entorhinal activity pattern provides only a partial or noisy version of the corresponding original pattern. Using this cue, the hippocampus reinstates the original pattern in the entorhinal cortex and, subsequently, the rest of neocortex. This operation is referred to as pattern completion, or autoassociative memory function. 7 Finally, the hippocampus may also be capable of autonomously reactivating stored memory traces in the absence of specific retrieval cues, thereby reactivating complete cortical memory representations during “off-line” behavioral states (e.g., slow-wave sleep). Such replay may contribute in various ways to the consolidation (transfer to a final repository) and maintenance of episodic and semantic memory (Kali & Dayan, 2004). But do we have any reason to believe that the hippocampus is even capable of carrying out these operations, and if so, that it is in some sense optimized for exactly these tasks? The most widely-cited evidence is the existence of the extensive recurrent collateral network of pyramidal neurons in area CA3. Such recurrent networks are the classic examples of autoassociative memory devices, whose properties have been extensively investigated by theoretical means and computer simulations (Marr, 1971; Willshaw & Buckingham, 1990; McNaughton & Morris, 1987; Treves & Rolls, 1992; Samsonovich & McNaughton, 1997; Kali & Dayan, 2000). In particular, the storage capacity of such networks, i.e., the number of patterns they can store and retrieve reliably, has been determined (Treves & Rolls, 1992), and was found to depend substantially on the properties of the set of input patterns that we attempt to store. Capacity is roughly inversely proportional to the sparsity of individual patterns (i.e., the proportion of active units in a pattern), and generally increases as the overlap between different stored patterns decreases. Therefore, it is reasonable to assume that the need to maximize storage capacity is an important reason for the conspicuously low activity levels of principal cell populations in the hippocampus (the proportion of active principal cells in any hippocampal subfield at any given moment is thought to be on the order of a few percent (Barnes et al., 1990; Quirk et al., 1992; Jung & McNaughton, 1993, Treves, 1994 #5013). 8 However, activity patterns in most areas of neocortex, including entorhinal cortex, appear to be much denser (involving a larger proportion of neurons at any given time), which is thought to be beneficial for generalization (McClelland et al., 1995), an important characteristic of the kind of representational learning that the neocortex may be engaged in. On the other hand, if the optimal type of activity pattern (dense vs. sparse) is different in the neocortex and the hippocampus, then the information contained in the entorhinal dense code needs to be “translated” into a sparse code when hippocampal representations are created (and vice-versa). In principle, such translation could be implemented directly by a single set of projections from the input area to the autoassociative network (i.e., by the direct perforant path input from entorhinal cortex to the CA3 region), without an intervening specific interface for sparsification. However, as argued on theoretical grounds by Treves and Rolls (1992), a projection with the characteristics of the perforant path – a large number of relatively weak, associatively -modifiable synapses on each target cell – may be optimal during retrieval, but a different type of input to the CA3 recurrent network – one with a small number of individually strong synapses per cell – is probably required for the storage of new memory traces. The mossy fiber pathway, the projection to CA3 from granule cells, meets the requirements for this second type of input. Additional theoretical and computer simulation studies by O'Reilly and McClelland (1994) indicated that the two-stage pathway from EC to DG to CA3 could perform very effective pattern separation, provided that individual mossy fiber connections were sufficiently strong to transfer the benefits of pattern separation in the DG to CA3. They further argued that the coexistence of this indirect pathway with the direct EC-CA3 connection enables the hippocampus to avoid an inherent conflict between pattern separation and pattern completion, both of which are important for the efficient operation of the 9 hippocampal autoassociator. In summary, these studies suggest that a possible role of the dentate gyrus is to form sparse, pattern-separated representations of entorhinal activity patterns, and transmit this sparse representation reliably for subsequent storage in the CA3 recurrent network. But how does the dentate gyrus create sparse representations from the relatively dense entorhinal activity patterns which constitute its only major cortical input? Since no direct experimental investigation of this issue has been undertaken, we need to rely on indirect evidence and the results of computational studies to try to answer this question. At an abstract level, well-known computational algorithms exist which create sparse, pattern-separated representations from distributed input patterns. A simple example is the competitive-learning pattern classification device described by Rumelhart and Zipser (1986), which has been shown to be capable of generating hippocampal place field-like activity patterns from input patterns resembling neocortical sensory representations (Sharp, 1991). This algorithm operates on binary input patterns and generates binary output patterns. For each presentation of an input pattern, the current values of the synaptic weights (which are initially set to random values) are used to determine the feedforward activation of units in the output layer. Then the output unit with the highest level of feedforward input is allowed to become active (this step is assumed to reflect the action of feedforward and feedback inhibitory circuits), and the incoming weights of this unit are allowed to change. This weight change is assumed to be Hebbian in nature: weights from active input units are increased, while weights from inactive units are decreased in such a way that the sum of all incoming weights remains constant (and identical to the sum of incoming weights to all other output units). This way, the “winning” unit will have an even higher level of feedforward activation the next time the same input pattern appears. It will also show an increased response to other 10 similar input patterns; on the other hand, its response to patterns which are very different (with a low degree of overlap) will diminish. As a result, different output units will eventually respond to different kinds of patterns, and end up partitioning the input space among themselves into non-overlapping groups of similar patterns. The algorithm performs both sparsification – since only a single output unit becomes active for any given (distributed) input pattern – and orthogonalization (pattern separation) – since relatively dissimilar, but still overlapping input patterns end up activating different output units (zero overlap). However, is there any experimental evidence that sparsification requires a separate relay station, and that the dentate gyrus has unique features - besides its well-known “detonator” type of terminals - for code conversion and reliable transmission? In the following pages we review behavioral, morphological, and physiological data relevant to these questions. Lesion studies Let us first examine whether the putative role of the DG as described above is consistent with behavioral data on the effects of specific lesions. In general, we expect that some, but not all tasks that are sensitive to global hippocampal lesions will also be sensitive to more specific lesions of the DG, and might hope that the pattern of impairments that occur after selective DG lesions sheds light on the role of the DG in hippocampal processing. Most lesion studies have taken advantage of the fact that intrahippocampal injections of colchicine cause a fairly selective destruction of the granule cells of the DG, but other techniques, such as neonatal X-ray irradiation and adrenalectomy, which also cause a similar pattern of damage, have also been used. Perhaps the most consistent finding after DG lesions in rodents has been a severe impairment in the acquisition of the reference memory task in the 11 Morris water maze (Sutherland et al., 1983; McNaughton et al., 1989; Conrad & Roy, 1993; Xavier et al., 1999). Working memory versions of the task are also affected, although perhaps to a lesser extent (Xavier et al., 1999; Jeltsch et al., 2001). Reference and working memory performance in the radial arm maze are also compromised (McNaughton et al., 1989; Jeltsch et al., 2001). In some more recent studies, DG lesions were also found to cause impairment in a delayed-matching-toplace task, in a temporal task (Costa et al., 2005), and in a task which required the detection of metric distance change between objects (Goodrich-Hunsaker et al., 2005). Recently, there have been some attempts to test more directly the proposed contributions of the DG to hippocampal function. In particular, Gilbert et al. (2001) designed a short-term spatial memory task where the proximity of relevant locations could be varied systematically. They found that DG lesions impaired performance at small, but not at large separations, consistent with the role of the DG in spatial pattern separation. Lassalle et al. (2000) examined the consequences of selective and reversible inactivation of mossy fiber synapses in CA3 in mice during various stages of a reference memory task in the Morris water maze. They found that the mossy fiber input from DG to CA3 was essential during learning, but not during the retrieval phase of the task, or in the period directly following learning (the early stages of consolidation). Similarly, Lee and Kesner (2004) attempted to distinguish encoding and retrieval deficits during the acquisition of a navigation task in the Hebb-Williams maze following lesions of various type. They found that lesions of the DG impaired learning, but not retrieval, in this task; conversely, lesions of the perforant path input to CA3 affected retrieval, but not learning. In summary, the available behavioral data from rodents with lesions to dentate granule cells or their mossy fiber output are generally consistent with a 12 crucial role of the DG in providing input to area CA3 during the acquisition of allocentric spatial information, and provide some support for a more specific function in (spatial) pattern separation. So what are the morphological peculiarities of the dentate gyrus which support its role in orthogonalization and make it indispensable for proper CA3 function? Morphological arguments - Heterogeneous terminal types of the mossy fibers Let’s see first how Cajal described of the unusual terminals types of the mossy fibers: “…there arise either short and thick divergent appendages or quite long fine threads that end in a swelling. Thus, we reproduced here the arrangement (although less distinctly) that we described in certain branched fibers of the cerebellum the mossy fibers. Therefore without further ado let us apply the same name to the axons of the granules of the fascia dentata.” It is clear from the above description that the investigator who named the mossy fibers by examining Golgi-stained material clearly identified that this peculiar fiber system has more than one terminal type. The well-recognizable giant endings (the large mossy terminals) gave rise to thin filamentous structures which ended in terminal-like swellings. Apparently the name “mossy fiber” is based on the presence of these filopodial extensions. Later electron microscopic work identified that filopodial terminals indeed establish asymmetrical synapses (Amaral, 1979; Claiborne et al., 1986) (Figure 1). In addition, a third terminal type has been described, small “en passant” boutons which resemble the most the conventional axonal varicosities of cortical pyramidal cells (Claiborne et al., 1986) (Figure1). Together the two smaller mossy terminal types far outnumber their larger counterpart, but still, the total number of terminals is actually very low (Claiborne et 13 al., 1986; Acsady et al., 1998). A single granule cell has no more than ~200 terminals, which is at least two orders of magnitude less than the number of varicosities along the axonal arbor of a cortical pyramidal cell. How can these few but variable terminals account for the code conversion and efficient transmission as outlined above? Unique features for code conversion The computational studies summarized above suggested that memory formation in the hippocampus may be a two-step process: the sparsification of the entorhinal signal by granule cells, followed by an association of the now sparse code in the CA3 network. Modeling studies suggest that the “winner-take-all” method of sparsification requires the recruitment of strong feedback inhibition. According to this scheme, the output of a small population of granule cells that become active in a given environmental context (e.g., during a couple of theta cycles as the rat passes through their place fields) activate GABAergic interneurons which exert fast and strong feedback inhibition on the somata and dendrites of the non-coding granule cells, shunting their entorhinal inputs and precluding their firing. As a result, synaptic plasticity only takes place at the perforant path input of the coding (granule) cells. However, feedback inhibition is well-known in all cortical regions. Is there any reason to suppose that feedback inhibition is more powerful in the dentate gyrus than in other cortical regions, which makes it especially useful for pattern separation? In cortical regions, interneurons constitute 10-20% of all the neurons. Cortical pyramidal neurons innervate their postsynaptic principal and interneuron targets in a quasi-random manner, i.e., the incidence of the targets is determined by the relative distribution of the neuron types (Gulyas et al., 1993; Sik et al., 1993). 14 Thus, the estimated ratio of interneurons among the postsynaptic targets of cortical pyramidal cells is around 10%. In the dentate gyrus granule cells have axon collaterals only in the hilus below the granule cell layer not in stratum granulosum or str. moleculare (Claiborne et al., 1986; Acsady et al., 1998). As a consequence only inhibitory neurons having somata and/or dendrites in the hilus can participate in feedback inhibition . Close to 50% of the hilar neurons are GABAergic (Houser & Esclapez, 1994) which suggests that inhibitory cells may be abundant among the postsynaptic targets of granule cells. The 5-8 hilar collaterals of the granule cells possess around 7-12 large mossy boutons and 102-147 small terminals (filopodial and en passant boutons) (Acsady et al., 1998). The postsynaptic targets of the small terminal types are almost exclusively interneurons, whereas targets of the large mossy terminals are mainly excitatory mossy cells (Acsady et al., 1998) (Figure 2). Thus, due to the surprising target selectivity of granule cell terminal types, interneurons may constitute up to 90% of the postsynaptic targets of the mossy fibers in the hilus, in contrast to the 10-20% GABAergic targets in other cortical regions. Many of these hilar neurons provide feedback inhibitory control of the granule cells, suggesting that proportionally stronger feedback inhibition is recruited here than in other cortical regions. A characteristic cell type of the hilus, the somatostatin-immunoreactive interneuron, provides a good example of the strong recurrent inhibition operating in this system. The somatostatin-containing hilar neurons restrict their entire dendritic arbor to the hilus and innervate the dendritic segment of granule cells in the zone where entorhinal afferents terminate (hence they are also called HIPP cells, i.e., HIlar interneurons with Perforant Path associated axon terminal) (Han et al., 1993; Sik et al., 1997). Unlike many other interneuron types that are characterized by a smooth 15 dendritic surface, the dendrites of HIPP cells are densely covered with thousands of long thin, elaborated spines (Baude et al., 1993). In contrast to the compartmentalized spines of the pyramidal cells, which usually receive a single terminal, these spines are contacted by multiple asymmetrical synapses (up to 8-10) (Acsady et al., 1998). Thus, these spines increase the total synaptic input of the HIPP cells enormously. Small terminals of granule cells have been described to contact these spines (at least 30% of the total synaptic output of granule cells contacts HIPP cells) (Acsady et al., 1998), whereas the axons of CA3 pyramidal cells that project back to the hilus selectively avoided HIPP cells (Wittner et al., 2006), suggesting that the majority of the excitatory input of these cells originates from granule cells. Since the number of contact between a granule cell and a HIPP cell is only 1 or 2 these morphological data indicate the convergence of several thousand granule cells on any one of these interneurons. The axons of HIPP cells terminate in the same layers as the perforant path , and therefore are in a critical position to modulate the information transfer from the entorhinal cortex to the dentate gyrus (Han et al., 1993). A single HIPP cell may form an unusually large number of axon terminals in the molecular layer (up to 80,000 compared to 5000-10000 in the case of a basket cell), the vast majority of which innervate granule cells (Sik et al., 1997). The little data available about the activity of HIPP cells suggests that these neurons are not fast-firing cells; rather, their activity is principally driven by the firing pattern of granule cells, a key feature for appropriately timed feedback inhibition (Buckmaster & Schwartzkroin, 1995). A second unique morphological feature of the connectivity in dentate gyrus is the lack of interaction among the major inhibitory basket cell classes and among basket cells and other interneurons (Acsady et al., 2000). In other hippocampal regions as well as in other cortical regions, basket cells densely innervate other 16 basket- and non-basket-type interneurons (Sik et al., 1995; Cobb et al., 1997; Tamas et al., 1998) forming an interacting local GABAergic network. For example, in the CA1 region the somatic region of parvalbumin-positive basket cells is contacted by more GABAergic terminals than the somatic region of pyramidal cells (Gulyas et al., 1999; Megias et al., 2001). In sharp contrast , GABAergic cells in the hilus of the dentate gyrus receive, on average, 15-40 times less input from local basket cells than mossy cells, the principal excitatory cell type of this region (Acsady et al., 2000). Since GABAergic cells inhibit each other, this connectivity pattern suggests minimal disinhibitory influence in the hilus and different GABAergic network dynamics. But what are the physiological properties of the granule cell-interneuron synapses? Examination of synaptic transmission at the granule cell-basket cell synapses demonstrated very fast kinetics: the postsynaptic conductance of the unitary current demonstrated submillisecond rise and decay (Geiger et al., 1997). This effect was largely attributed to the high synchrony of transmitter release and the rapid time course of AMPA receptor deactivation. The fast postsynaptic response allows rapid activation of feedback inhibition, which supports a role in pattern separation. These data suggest that the basic principles of connectivity between excitatory and inhibitory neurons in the dentate gyrus are significantly different from those in any other cortical region. The peculiar arrangement of excitatory and inhibitory connections in the dentate gyrus suggests an unusually strong recruitment of inhibition that can be used to suppress the activity of granule cells in a competitive manner. As a result, only granule cells with the strongest entorhinal excitatory drive will participate in the information transfer to CA3. This small population of active granule cells could effectively prevent large granule cell populations from reaching firing threshold via the strong feedback inhibitory system outlined above. Only those 17 entorhinal inputs will be potentiated which contact active granule cells. This would further strengthen the competitive process, which could lead to the sparsification of the entorhinal signal resulting, e.g., in the sharp and focused place fields of the granule cells which are in sharp contrast to the grid-like entorhinal signal (see below). The next step is to faithfully transmit this recoded cortical signal to the associational station, the CA3 region, and to ensure that only the activated subset of CA3 pyramidal cells would be included in the autoassociation network responsible for memory storage. Unique features for efficient and sparse transmission The giant mossy terminals display all the morphological features of a classic “detonator” or “driver” type terminal. No other cortical synapse is comparable to them, but several subcortical structures (e.g., thalamus, cerebellum) utilize similar synaptic arrangements to secure faithful synaptic transmission (Sherman & Guillery, 1998). A single large mossy terminal may establish up to 30-40 release sites, all converging on the proximal dendrite of a single postsynaptic pyramidal cell (Chicurel & Harris, 1992; Acsady et al., 1998). One would predict that the short electrotonic distance from the soma would maximize the efficacy of the input in driving the postsynaptic cell to threshold. Recent in vitro and in vivo data confirm this assumption. Monosynaptic AMPA/kainate receptor-mediated EPSCs from granule cell-pyramidal cell pairs had a mean peak amplitude of -163.0 ± 23pA at 70mV in organotypic slice cultures which displayed morphological properties similar to the in vivo condition (Mori et al., 2004). Strong excitatory action has been described earlier between granule cells and their excitatory targets in the hilus, the mossy cells (Scharfman et al., 1990). In the in vivo anesthetized preparation, repetitive firing in a single granule cell reliably induced action potentials in 18 monosynaptically connected CA3 pyramidal cells (Henze et al., 2002). It has to be emphasized that such strong coupling is extremely rare among excitatory cells in cortical circuits. The general rule is that a large number of excitatory inputs have to be simultaneously active to reach postsynaptic spike threshold. But how sparse is the detonator signal in morphological terms? The number of large mossy terminals along the single unbranching axon of granule cells within area CA3 is very low (average:12.3; range: 10-18) (Acsady et al., 1998). If one considers that a single CA3 pyramidal cell may have up to 60 000 terminals, it is straightforward to conclude that granule cells are a specific cortical cell type designed to transfer the sparse code generated in dentate gyrus very effectively to only a restricted set of postsynaptic pyramidal cells. Two recent studies described additional surprising features of the mossy fibers. These features not only support faithful transmission through this pathway, but also demonstrate the computational power of the axon terminals in unexpected ways. The first study (Engel & Jonas, 2005) describes the active properties of the large mossy terminals, which facilitates reliable transmission of high frequency trains of action potentials. Apparently, mossy terminals have a very high density of specialized Na+ channels with faster activation and inactivation kinetics than somatic Na+ channels. These Na+ channels enable reliable action potential invasion into large mossy terminals and increase presynaptic Ca2+ influx, resulting in up to 16-fold increase of transmitter release. In addition, modeling studies suggest that this active property of the terminals is absolutely necessary to induce Ca2+ influx into the filopodial extensions to trigger glutamate release at the mossy fiber-interneuron synapse. This mechanism may underlie the high release probability of the filopodial connection compared to the mossy fiber- CA3 pyramidal cell synapse (Jonas et al., 1993; Lawrence et al., 2004). 19 The second study (Alle & Geiger, 2006) demonstrates that mossy fibers transmit not only action potentials, but also postsynaptic potentials originating in the soma-dendritic compartment. These “excitatory presynaptic potentials” alter the transmitter release of the subsequent action potential (within a 10-20 ms delay), thus enabling the axon to integrate subthreshold and suprathreshold signals provided they are temporally proximal. In sum, mossy terminals fulfill all criteria for a classical, sparse “detonator” synapse. The strong granule cell-pyramidal cell connection, however, poses two major problems for the autoassociative network of the CA3 region. Problem 1) Spontaneously-active granule cells, which represent only background noise, may induce irrelevant spiking of CA3 pyramidal cells, resulting in non-coding CA3 networks which may overlap with the coding networks. Problem 2) Spontaneously-active CA3 pyramidal cells may generate action potentials coincident with the CA3 spikes evoked by granule cell firing, which represent the given environment. These non-coding EPSPs will be also strengthened in the autoassociative CA3 network, which would ruin the orthogonalized, sparse code. Selective activation of the CA3 network participating in coding – solution to problem 1 and 2. A first solution to problem 1 is that granule cells have very low spontaneous firing rates (0.1-0.01 spike/sec) (Jung & McNaughton, 1993), probably due to their hyperpolarized resting membrane potential (-80 mV in vivo) (Penttonen et al., 1997) and/or strong inhibitory control. Still, if we consider that there are one million granule cells per hippocampus in rodents (Seress, 1988) and we take the low end of their spontaneous discharge frequency 20 (0.01 Hz), we can calculate that at least 10 granule cells will fire in any one millisecond, activating ~120 CA3 pyramidal cells, each of which has around 30 000 terminals within the CA3 (Li et al., 1994). Thus, other solutions are needed to solve problem 1. Similar to the hilus, the number of small mossy fiber terminals in the CA3 region exceeds the number of large mossy fiber terminals by a factor of at least four (Acsady et al., 1998). As in the hilus, the small terminals selectively innervate inhibitory cells. All examined inhibitory cell classes (perisomatic, dendritic and interneuron-selective) were among the postsynaptic elements of granule cells. Since a single mossy fiber rarely innervates a postsynaptic interneuron via multiple contacts (Acsady et al., 1998), granule cell firing activates at least four times as many inhibitory as excitatory cells. Physiological data confirm strong activation of this feedforward inhibitory circuit. In paired recordings, a single action potential in the granule cell induced a biphasic response in the postsynaptic CA3 pyramidal cell: a brief EPSC followed by a pronounced IPSC (Mori et al., 2004). The direction of the summated charge transfer was outward, indicating a net inhibitory synaptic response. The authors calculated that approximately four interneurons fired together to evoke the measured inhibitory responses, which corresponds well to the anatomical data. Reliable activation of interneurons was also observed in vivo. Multiple granule cell spikes induced firing in monosynaptically-coupled interneurons with quite high probability (Henze et al., 2002)(Figure 3D), suggesting that although the granule cell-interneuron contact rarely contains multiple release sites, the single active zone of the small terminals is still efficient enough to induce postsynaptic firing of the GABAergic cell. Recruitment of more inhibitory than excitatory cells suggests that the net effect of the excitatory mossy fiber system on the CA3 pyramidal cell population is, counter-intuitively, inhibitory. 21 A peculiar EEG transient, the dentate spike, can be utilized to demonstrate the impact of granule cell activation on the postsynaptic cell populations along the dentate-CA3 axis during a “natural” stimulus. Dentate spikes are short-duration, large-amplitude field potentials caused by synchronous activation of the entorhinal input, which occur during behavioral immobility and slow wave sleep (Bragin et al., 1995). Extracellular recordings during dentate spikes demonstrated increased unit activity in hilar neurons (many of which are GABAergic), but suppressed multiunit activity in the CA3 region (Bragin et al., 1995). Intracellular studies confirmed depolarization of granule cells and hilar interneurons but hyperpolarization in CA3 and CA1 pyramidal cells (Penttonen et al., 1997). It is worth mentioning that this pattern of activity is in sharp contrast to the neuronal behavior that can be observed during the other major excitatory field transient in the hippocampus, the sharp wave, which originates in CA3. Since in this case there is no inhibitory “barrier” comparable to the dentate GABAergic network which could block the spread of excitation, sharp waves propagate not only to the CA1 region but also to parahippocampal cortical regions via polysynaptic activation (Chrobak & Buzsaki, 1994; Chrobak & Buzsaki, 1996). Most recently the efficacy of the dentate inhibitory “barrier” was demonstrated by comparing the correlation of intracellular activity of neurons in various cortical fields with the UP and DOWN states in the EEG during slow cortical oscillation (Isomura et al., 2006). Surprisingly, all cortical regions (including entorhinal cortex and dentate gyrus) changed intracellular activity coherently with the EEG states with the exception of CA3 region. In contrast to basically all cortical cells CA3 pyramidal cells were not active during the UP states. Apparently the inhibitory network launched by the dentate during the 22 UP states is able to shield CA3 even from the most synchronous excitatory events of the cortical mantle. In summary, anatomical and physiological data provide convergent evidence for the conclusion that the output of the granule cells activates an unusually strong feedforward inhibition to area CA3. Since even a single basket cell is able to block action potential generation in a large number of pyramidal cells (Miles et al., 1996), feedforward inhibition will effectively reduce the number of active CA3 pyramidal cells during dentate activation. In addition, in the CA3 region, specialized interneuron types exist which restrict their dendritic (Gulyas et al., 1991) or axonal (Vida & Frotscher, 2000) arbor to stratum lucidum of CA3, suggesting selective control of this pathway. Thus, the likely solution of Problems 1 and 2 is that “background” or “non-coding” EPSPs and action potentials in CA3 are actively inhibited during dentate-CA3 information transfer by the strong feedforward inhibition. In this way only the small population of CA3 cells receiving the decorrelated sparse dentate signal will be active, and, following the Hebbian rule, only the recurrent synapses between the activated CA3 cells will be potentiated. According to the modeling studies (see above) the matrix of potentiated synapses will represent the memory trace. However, what happens to the EPSPs generated by the spontaneous activity of CA3 pyramidal cells immediately before the dentate input arrives? The EPSPs among pyramidal cells are quite slow (half duration, 27 ms) (Miles & Wong, 1986) and accidentally the mossy fiber input can arrive together with their peak, thus these early background (“unwanted”) EPSPs can be potentiated before the disynaptic feed forward inhibition arrives. A recent report provides a possible solution for this problem (Kobayashi & Poo, 2004). This study describes LTP at the recurrent synapses of the CA3 network induced by paired stimulation of mossy fiber 23 input and the associational/commissural input. First, this study provides direct and elegant evidence for the role of mossy fibers in changing the synaptic strength of the autoassociative CA3 matrix. Second, an interesting observation from this study helps resolve the problem of early EPSPs. The results demonstrate that the potentiation of associational input depends on the relative timing of mossy fiber and associational spike trains. The potentiation was smaller if additional associational spikes were added before the paired stimulation, compared to the protocol that added spikes after the paired (associational/mossy fiber) pulses. The effect depended on mGluR1 activation. Thus, apparently the system favors the potentiation of CA3 EPSPs arriving coincidentally or after the dentate signal. Since the mossy fiber input is able to induce CA3 spiking in vivo, these EPSPs will mostly represent the spiking of CA3 pyramidal cells evoked by the sparse, orthogonalized dentate input. In this way only the associational EPSPs representing dentate activity will be potentiated, but EPSPs arriving earlier, representing spontaneous CA3 activity, will not. Target-dependent plasticity - the meaning of various terminals types If we consider the strong feedforward inhibition operating along the dentate-CA3 axis the following, third problem arises: Problem 3: How to overcome the strong feedforward inhibition when the specific dentate pattern has to activate the pyramidal cell? Apparently the solution to Problem 3 is that short- and long-term plasticity at large and small types of mossy fiber ending are different. The physiological data clearly demonstrate that the small terminals are not only distinct morphological units, evolved to contact a large number of interneurons, but discrete compartments which harbor distinct molecular machinery for plasticity different from their giant cousins. 24 Small and large mossy terminals display different types of short-term plasticity. The giant mossy terminal-pyramidal cell connection express very strong short-term facilitation (Salin et al., 1996; Toth et al., 2000), reaching up to threefold amplitude increase on average at the fifth EPSC in case of 20 Hz stimulation. (This is highly unusual in the case of giant “driver”-like terminals; e.g., in the thalamus excitatory terminals with a similar synaptic arrangement show strong depression in relay cells (Reichova & Sherman, 2004)). In contrast the small mossy terminalinterneuron connection hows short-term depression after repetitive mossy fiber stimulation in about 50% of the interneurons. Others show modest facilitation at 20 Hz (Toth et al., 2000), but at 40 Hz, all responses are depressed after the 8th action potential (Mori et al., 2004). The third synapse in the feedforward inhibitory circuit, the interneuron-CA3 pyramidal cell contact, expresses pronounced short-term depression (Mori et al., 2004) at all frequencies tested. This variability in short-term plasticity at different mossy fiber synapses favors conditions for a single granule cell action potential to induce weaker excitation, but relatively strong feedforward inhibition. Repetitive firing, however, rapidly increases the effect of excitation and at the same time decreases the efficacy of inhibition. Thus, the net effect of mossy fiber activation on CA3 pyramidal cells depends heavily on the frequency of granule cell firing. The system appears to act as a high-pass filter, where spike transmission from granule cell to pyramidal cell is blocked at low frequencies but favored when the frequency of granule cell discharge increases. Recently this assumption was tested directly in in vitro paired recordings of granule cells and pyramidal cells (Mori et al., 2004). The postsynaptic potentials evoked in pyramidal cells by granule cell stimulation were measured at increasing frequencies (10-40 Hz). The inhibitory dominant PSPs observed during low frequency trains switched to excitatory dominant PSPs at high frequencies. At 40 Hz 25 EPSPs dominated the response already after the third granule cell action potentials, whereas at 10 Hz the response remained inhibitory even at the 15th action potential. Frequency-dependent facilitation of mossy fiber transmission was demonstrated in monosynaptically-coupled granule cell - pyramidal cell pairs in vivo as well (Henze et al., 2002) (Figure 3). The probability of postsynaptic pyramidal cell spikes rapidly increased with increasing granule cell firing and reached 0.8 at 100 Hz (Figure 3E), which is an extremely high value in cortical circuits and results in an almost one-to-one relay of the granule cell activity. Within the spike train the probability of CA3 pyramidal spikes increased with the number of presynaptic granule cell spikes. The maximum spike transmission probability was reached after the 4-5th spike (Figure 3F). In contrast, in the case of interneurons, the probability of transmission did not increase with an increasing number of presynaptic spikes at 100 Hz and remained lower than that of the pyramidal cell (Figure 3F). These in vitro and in vivo data indicate that differential short-term plasticity in this feed-forward circuit result in a frequency-dependent shift of the polarity of postsynaptic response. In the freely-moving condition, granule cells have very low spontaneous firing rates, which can rapidly increase to 40 Hz (Jung & McNaughton, 1993) as the animal enters the place field of the neuron. As a consequence of the frequencydependent switch from excitation to inhibition described above, the probability of spike transmission between the DG and CA3 is very low at low firing rates, despite the “detonator” nature of the synapse. When granule cells code the specific information of the environment, the probability of spike transmission to CA3 becomes very high. Thus, granule cells can act as a “conditional detonator” as suggested by Henze et al. (2004). This mechanism solves both the problem of filtering out non-coding spontaneous activity (Problem 1), and resolves the issue that 26 strong feedforward inhibition must be overcome when specific information must be transmitted from dentate to CA3 (Problem 3). The delicate frequency-dependent balance between excitation and inhibition substantially increases the computational power of the mossy fibers. It raises the possibility that they not only participate in the faithful transmission of an orthogonalized dentate signal but they themselves participate in creating the nonoverlapping representations in the CA3 region. Recent data suggest (Leutgeb et al., 2006, see below) that the representation of the environment is more orthogonalized in CA3 than in the dentate gyrus, which questions the role of dentate as the sole contributor to this process. Due to its high-pass filter nature, the dentate-CA3 circuit may refine the dentate code, and, e.g., participate in creating the single receptive field observed in CA3 place cells as opposed to the multiple place fields of dentate place cells. The critical variable in this process will be the exact firing pattern of the dentate granule cell. For instance, assume that a granule cell fires at 40 Hz in one of its receptive fields but only 10 Hz in the other. Based on the data discussed above, only the higher firing rate will induce firing in the CA3 pyramidal cell, the lower activity will be filtered out, and the CA3 pyramidal cell will display a single receptive field as a result. What about long term changes? One presentation of the dentate code may not be sufficient to induce long-term changes in the CA3 recurrent network. Multiple presentations (e.g., crossing the place field several times) or autonomous replay of the memory trace in absence of the original condition, may be necessary to induce long-term plasticity. Replay of a given firing pattern may occur during different EEG states, as has been shown for theta and sharp wave activity (Nadasdy et al., 1999), or during the subsequent sleep episodes (Wilson & Mcnaughton, 1993). 27 Tetanic stimulation of mossy fibers induces long-term potentiation in pyramidal neurons, but is either without effect, or it induces depression at synapses onto interneurons (Maccaferri et al., 1998). Since the mossy fiber-LTP onto pyramidal cells critically depends on cAMP, the effect can be explained by the absence of the adenylyl cyclase-cAMP cascade from the filopodial and “en passant” small terminals. As a result of this differential LTP, the critical frequency at which inhibition switches to excitation may change and/or the steepness of the high-pass filter cut-off may increase. In this way, presynaptic mossy fiber -LTP may help the orthogonalization process performed by the dentate-CA3 circuit and creates a good opportunity for the faithful activation of the same CA3 circuit in case of repetitive presentation. Finally, morphological plasticity of the small terminals provides yet another way to fine-tune the balance of excitation and inhibition in the mossy fiber pathway. The structure of the filopodial terminals strongly resembles rapidly advancing and retracting axonal filopodia observed during axonal development and synapse formation. Recent studies of slice cultures indeed demonstrated that over one-third of the filopodia are highly active (De Paola et al., 2003; Tashiro et al., 2003). In addition, in mature slices, approximately 9% of the small en passant boutons were also labile (half-life, approximately 1 day), in contrast to the stability of large terminals . Interestingly, in mature cultures, the total number of synapses remained stable in the presence of substantial turnover of individual terminal structures. Motility of the filopodial extensions was observed not only in slice cultures but also in the acute whole-mount hippocampal preparation and acute hippocampal slices. This actin-based motility can be regulated by brain-derived neurotrophic factor (BDNF), AMPA and/or kainate receptors, in a cAMP dependent manner (De Paola et al., 2003; Tashiro et al., 2003). These data strongly suggest that target selectivity of 28 the small terminal types of the mossy fiber is based on the dynamic morphological properties of these axonal elements. In addition, they suggest that even in mature animals activity change, traumatic injury or cell loss may induce rapid remodeling of the mossy fiber-to-interneuron connections from granule cells. Indeed, ischemic damage, which results in the loss of hilar and stratum lucidum interneurons, dramatically reduces the number of filopodia (Arabadzisz & Freund, 1999). In summary, the mossy fiber pathway maximally utilizes the opportunity to differentially regulate its postsynaptic partners. Selective innervation of pyramidal cells and interneurons by distinct terminal types creates a favorable condition to differentially regulate short-term and long-term plasticity and the motility of various mossy terminal types. The “bottom-line” of this highly dynamic system appears to be the fine-tuning of the balance between the excitation evoked by the large “detonator” terminals and the feedforward inhibition activated by the small terminals. This will determine exactly which dentate firing patterns will induce permanent modification of the autoassociational network of the CA3 region. The final test for all predictions regarding dentate function is the examination of firing activity in the freely-moving animal. Neuronal activity patterns in vivo and the processing of entorhinal input by the dentate gyrus Compared to the vast amount of data available on spatial (and non-spatial) representations in area CA1 of the hippocampus, relatively little is known about the in vivo firing properties of neurons in the dentate gyrus under various behavioral conditions. During exploration, granule cells display spatially-selective activity, and their firing behavior is, at least under most conditions studied so far, qualitatively 29 quite similar to that of pyramidal cells (“place cells”) in areas CA1 and CA3. In particular, in the radial arm maze, granule cells have directional place fields, although the average number of subfields is somewhat higher and the average size of these subfields is slightly smaller than in CA3 place cells (Jung & McNaughton, 1993). The firing activity of dentate granule cells is modulated by local field potential oscillations in the theta frequency range, and the timing of individual action potentials changes during traversals of the place field similar to phase precession observed in CA1 pyramidal cells (Skaggs et al., 1996). When different spatial cues were put in conflict by manipulating the environment, sudden coherent transitions (known as reference frame shifts) could be observed in the activity of the set of simultaneously-recorded granule cells, also analogous to the behavior of the CA1 cell population under these conditions (Gothard et al., 2001). In an experiment where an explicit attempt was made to identify non-spatial as well as spatial responses, a subpopulation of granule cells showed position-selective or position-independent reward site responses, whereas another population showed pure place responses (Tabuchi et al., 2003). When rats are allowed to explore a novel environment for the first time, both dentate granule cells and CA1 pyramidal cells acquire distinct spatial preferences within the first few minutes (Nitz & McNaughton, 2004). However, concurrentlyrecorded interneurons showed very different behavior in the two regions: while most CA1 interneurons transiently decreased their activity while the animal was exploring a novel environment, the majority of interneurons in the DG significantly increased their activity during the same epoch. These data are congruent with the role of strong feedback inhibition in establishing sparse and decorrelated output in the DG. In order to understand the nature of the computations performed by the DG, it is essential to have an accurate description of the cortical input it receives. The last 30 few years have witnessed a major revolution in our understanding of spatial representations in entorhinal cortex. Until a few years ago, existing data on EC representations indicated that, although neurons in EC were spatially selective, their place fields were much larger and less clearly defined than those in hippocampal areas (Quirk et al., 1992; Frank et al., 2000). Indeed, this was perhaps the most direct experimental evidence for the assumption that sparse, orthogonal, "hippocampaltype" representations are created first in the dentate gyrus. However, when spatial firing patterns were measured in the part of medial entorhinal cortex (mEC) which projects to the dorsal HC (where place fields are normally recorded), much smaller place fields, similar in size to corresponding hippocampal place fields, were found (Fyhn et al., 2004), while areas of mEC with large place fields projected to more ventral parts of the HC, which itself was found to have large place fields (Maurer et al., 2005). From these data, it appeared that there was in fact no major transformation of spatial representations from EC to the DG (and the rest of the hippocampus), although subtler differences (especially in response to environmental manipulations) could not be ruled out. However, it soon emerged that if spatial firing patterns are recorded over a larger spatial scale, entorhinal and hippocampal representations are again fundamentally different. In particular, EC place fields were found to repeat periodically at the vertices of a regular hexagonal lattice, and different EC "grid cells" differ in the center location ("phase"), orientation, and scale of the grid (Hafting et al., 2005). In contrast, hippocampal place cells have at most a few discrete place fields even in these larger environments, and their fields typically do not have any special geometrical relationship. Thus, it currently appears that hippocampal spatial representations are in fact different in nature, and, in particular, much sparser over a large environment than representations in the (medial) entorhinal cortex, and a transformation (in fact, a pattern separation operation) by the 31 DG is still required. Indeed, if we consider two locations in the environment which are separated by the grid period (assumed to be relatively invariable) in the area of entorhinal cortex which projects to a given part of the HC, the activity of the grid cell population will be quite similar, while the hippocampal activity pattern will be distinct, reflecting the outcome of some kind of pattern separation process. In fact, the need for pattern separation becomes even more obvious if we consider two environments. The root of the problem is the observation that the relative grid parameters (phase and orientation) of different grid cells appear to be fixed in all environments, so, in principle, there must be corresponding locations (and orientations) in two distinct environments where the activity of the entire grid cell population is identical. This raises an obvious question: how distinct spatial representations of the two environments could be formed in the HC based on a single entorhinal grid cell representation. One possible answer to this question is based on the fact that the entorhinal grids are not perfectly regular; for instance, peaks in the grid vary in amplitude, so that, in principle, there could be sufficient spatial information present in the amplitudes to distinguish different environments. Another, perhaps more plausible explanation is that the hippocampus (and, in particular, the dentate gyrus) receives, in addition to input from medial EC, where grid cells are located, input from lateral EC, where neuronal firing patterns have a lower spatial information content, but, instead, carry more information about relevant objects (Hargreaves et al., 2005). Such object-based information is probably sufficient to disambiguate different environments, and create distinct codes in the HC. Hippocampal pattern separation between environments of varying degrees of similarity has recently been investigated in a series of experiments (Leutgeb et al., 2004; Leutgeb et al., 2005c; Leutgeb et al., 2005b), and the initial analysis in areas CA3 and CA1 has now been partially extended to mEC and the DG (Hafting et al., 32 2006; Leutgeb et al., 2005a; Moser et al., 2006). By analyzing the spatial firing fields of neurons within environments of varying degrees of similarity (manipulations included switching between different recording locations, as well as changes in the shape and/or the color of the enclosure), Moser and colleagues found that firing patterns of neurons in all hippocampal areas distinguished between different environments much better than those of grid cells in mEC. Essentially no pattern separation was detected in EC, as population firing patterns in enclosures of different shapes, colors, or even in different rooms were not significantly different (any observed changes occurred coherently in all recorded neurons; (Hafting et al., 2006)). However, they also found that the basic properties of pattern separation were different between different subfields of the hippocampus. In area CA3, the phenomenon termed "rate remapping" was observed when the shape of the enclosure was varied continuously: the firing rate pattern of the active cell population changed continuously as the environment was gradually transformed, while place field locations remained constant (Leutgeb et al., 2005b). In the end, environments of clearly distinct shapes (e.g., circle vs. square) activated CA3 pyramidal cell populations with relatively little overlap - indeed, when recordings were made in two different rooms, the two populations of active CA3 neurons appeared to be chosen independently, a phenomenon referred to as "global remapping" (Leutgeb et al., 2005c). Interestingly, preliminary data from recent experiments have revealed a radically different type of pattern separation in the DG. First, unlike in CA3, the same dentate cells were found to be active in different environments (Moser et al., 2006). Second, DG neurons typically had multiple place fields even in a single environment, consistent with earlier data (Jung and McNaughton, 1993). Finally, the peak firing rate within any one place field of a single cell varied with even small 33 changes in the shape of the enclosure, independently of rate changes in other fields of the same cell (Leutgeb et al., 2005a). These results show that the DG performs a pattern separation operation on its entorhinal inputs, but pattern separation appears to work differently from what was previously assumed. The observation that the proportion of simultaneously active cells is much lower in the DG than in EC (a fact that was confirmed by the data described above, and independently by another recent study, which measured immediate early gene expression in the DG following spatial experience;(Chawla et al., 2005)) suggested that different environments might activate different sets of neurons in the DG, thereby implementing a particularly efficient type of pattern separation. The results of Moser and colleagues now suggest that this might not be the case, and different environments (as well as different locations within these environments) might be encoded by different firing rate patterns in essentially the same population of active DG neurons. This latter scheme potentially also allows a fine discrimination of different environments (and locations) based on the DG population activity pattern (especially since the firing rates of DG neurons appear to be rather sensitive to changes in environmental features). However, since the CA3 code appears to be more efficiently orthogonalized than the DG code, an additional processing step may be needed. The physiological data reviewed above indicate that the mossy fiber projection may be utilized to arrive at the sparser, more completely pattern-separated representation recorded in area CA3, but further contributions from other sources (temporo-ammonic pathway and local network connections) cannot be excluded. The different behavior of spatial representations at different stages of hippocampal processing clearly argues against the simple view that all properties of hippocampal place cells originate in the dentate gyrus, and downstream areas simply inherit these properties. A more complex view of the formation of hippocampal 34 representations is also indicated by recordings of neuronal activity patterns following selective lesions. In particular, following colchicine lesions of dentate granule cells as described above, place cell representations could still be observed in area CA1 (McNaughton et al., 1989). Similarly, the spatial representation in area CA1 was largely intact after surgical separation from all other hippocampal areas, which left it with the direct projection from entorhinal cortex as its sole cortical input (Brun et al., 2002). Therefore, hippocampal areas other than the DG must be capable of creating sparse, distributed, place-field-like representations on their own under some circumstances, probably based on their direct entorhinal inputs. However, it has to be kept in mind that despite the presence of a proper place cell representation in CA1, navigation memory was compromised following both types of lesion, suggesting that utilization of the spatial code at the behavioral level requires intact dentate-CA3 interaction. In summary, these data suggest that the DG does perform a pattern separation of the entorhinal signal, which, however, needs further processing to achieve the sparse and decorrelated activity pattern observed in the CA3 region. The dentate gyrus: an evolutionary-developmental perspective Apparently the basic organization of the dentate-CA3 network has the deepest phylogenetic root among cortical regions. Before the divergence of reptilian-mammalian lineages which apparently preceded the mass extinction of the Permian period (~250 million years ago) neurons in all cortical areas were most probably packed in a single cellular layer. During the ontogenesis they likely followed an outside-in pattern of histogenesis, where newly-generated neurons settle below the older ones, like in extant reptiles (Goffinet et al., 1986). Their main excitatory afferents entered and terminated in the embryonic marginal zone, above 35 the cortical plate, i.e., in the same zone where the apical dendrites of their main targets (excitatory principal cells) were present (Ten Donkelaar, 1998). As suggested recently, this arrangement does not support the evolution of a cortical structure with multiple cellular layers and distinct areas (Super & Uylings, 2001). As the mammalian nervous system evolved, the dorsal and lateral cortex of the ancient reptilian cortex underwent a significant modification that opened up tremendous opportunities for areal and cellular diversification (Super & Uylings, 2001). This included changing the pattern of histogenesis to the inside-out pattern, where newly generated neurons settle above the older ones, and changing the pattern of axonal ingrowth (for a review see Super and Uylings, 2001). In the mammalian neocortex, most of the afferents fibers enter not above but below the cortical plate, via the subplate (Allendoerfer & Shatz, 1994; Molnar, 2000). This new developmental scheme allowed the development of multilayered cortical structures with highly variable cell types, rich reciprocal interaction with the thalamus and the establishment of new cortical regions. Little developmental change occurred, however, in the medial and dorsomedial cortex, which became the mammalian allocortex. In the dorsomedial cortex that is homologous with the Cornu Ammonis, the pattern of histogenesis changed to the inside-out pattern, but the pattern of axonal ingrowth did not (Super et al., 1998). However, in the most “stubborn” structure, the dentate gyrus, the basic reptilian developmental pattern was retained (Bayer, 1980) histogenesis follows the outside-in pattern and the main excitatory afferents enter above the cortical plate. Neocortex has evolved rapidly to become a highly complex multilayered structure, with extensive intracortical and thalamo-cortical reciprocal connections (Butler, 1994; Nieuwenhuys, 1994; Rakic, 1995; Northcutt & Kaas, 1995). 36 Allocortex remained a unilayered structure with a basically unidirectional information flow, dentate gyrus being the only cortical structure without thalamic input (Amaral & Witter, 1989). Neocortex has been significantly expanded laterally and was parceled into numerous functionally segregated areas, but the hippocampus retained the original two major subfields, the dentate gyrus and Cornu Ammonis. For these two regions, only the Cornu Ammonis showed some areal segregation, and the original reptilian dorso-medial cortex became CA3, CA2, CA1 and subiculum (Amaral et al., 1990). Again, the dentate gyrus showed no areal segregation. But why did the DG-CA3 connection remain essentially unchanged during the course of evolution, when the rest of the cortical mantle underwent a dramatic reorganization? Here we would like to propose that the reason behind the protracted evolutionary pattern of the hippocampus, and especially the dentate gyrus, is the structural constraints of hippocampal function. Apparently, the formation of freely accessible multidimensional memory traces can only be performed in a two-step process that includes a segregation of input followed by an associative step. The reciprocally-coupled, multilayered cortical structures evolved for a different role (for a more complex interaction with the environment). Apparently, the basic plan of the two-step information processing through the hippocampal formation remained essentially the same from lizard to human. Similarly to mammals, in reptiles, the dentate-equivalent medial cortex receives the cortical input. This cortical region lacks an autoassociative network and projects the recoded information unidirectionally, to the reptilian analogue of the Cornu Ammonis, the dorsomedial cortex (Lopez-Garcia & Martinez-Guijarro, 1988; Martinez-Guijarro et al., 1991; de la Iglesia et al., 1994). Association functions in the reptile may take place in the next step here in the dorso-medial cortex, where an extensive recurrent collateral system exists, similar to the CA3 region in mammals (Martinez Guijarro et al., 37 1984). Interestingly, damage to the reptilian homologue of hippocampus causes similar learning problems as hippocampal lesions do in mammals (Rodriguez et al., 2002), suggesting an analogous structural-functional relationship. Has anything changed in dentate gyrus during the mammalian evolution? In primates, the volumetric ratio of the dentate gyrus and CA3 has changed in favor of the first. Indeed, dentate gyrus is “dentate” sensu stricto only in primates, where it includes numerous infoldings. Among these areas the hilus showed the largest relative increase in volume and cell number (Seress, 1988) underlying the importance of the region, where most of the peculiarities in microcircuits have been noticed. Apparently, the feed back regulation of granule cells became more elaborated with the increasing complexity of information to be categorized by the system. Conclusions, unresolved questions The dentate gyrus appears to be an ancient cortical structure from the phylogenetic perspective, yet it displays a number of unique features not found in other cortical regions. Its morphological and physiological properties are highlyspecialized, and these can explain, at least in part, the role assigned to the DG and CA3 by computational theories. In particular, mossy fibers are characterized by distinct mechanisms of signal transfer at excitatory vs. inhibitory targets, and unusually strong activation of GABAergic circuits. This arrangement allows a delicate balance of excitation and inhibition, which is utilized for code conversion and sparsification at the entorhinal-dentate connection and for frequency-dependent spike transfer at the dentate-CA3 connection. Several issues, however, remain unresolved. More comparable data are needed from freely-moving animals to understand the precise computation that takes 38 place in the DG and in CA3. Since DG-CA3 transmission appears to be dependent on the frequency of granule cell discharge, DG firing patterns should be carefully analyzed, and particularly with respect to multiple receptive fields. The role of two major excitatory inputs of the granule cells, not discussed here, the mossy cell input and the supramammillary afferents, is necessary to clarify DG information processing comprehensively. Both mossy cells and supramammillary afferents contact the proximal dendrites of granule cells, and therefore are likely to exert a powerful influence. Mossy cells of the hilus have highly divergent axons, and thus may link distant DG populations involved in coding similar environmental events, whereas the supramammillary input may mediate the modulation of granule cells by the theta rhythm. The role of rhythmic EEG activities (theta, gamma) in mediating signal transfer, code conversion, and the short- and long-term plasticity is unclear at the present time. Similarly, the role of dentate spikes is not explored. It is tempting to speculate that they may participate in memory replay, like the sharp wave in the CA3-CA1 network (Buzsaki, 1989), but definitive proof is currently unavailable. From the computational perspective, it is not clear how the lack of connectivity among granule cells and the relative paucity of the direct backprojection from the CA3 to the DG (Li et al., 1994) helps the categorization function in DG. In conclusion, this peculiar neocortex-archicortex interface will likely keep us busy for a long time. Acknowledgement: This work was supported by the Wellcome Trust (A.L. is the recipient of a Wellcome Trust International Senior Fellowship), the Institut de Cerveau et de la Moelle épiniere, the Hungarian Scientific Research Fund (OTKA T 049100) and the EU Framework 6. 39 List of references Acsady, L., Kamondi, A., Sik, A., Freund, T. & Buzsaki, G. (1998) GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci, 18, pp. 3386-3403. Acsady, L., Katona, I., Martinez-Guijarro, F.J., Buzsaki, G. & Freund, T.F. (2000) Unusual target selectivity of perisomatic inhibitory cells in the hilar region of the rat hippocampus. The Journal of neuroscience, 20, pp. 6907-6919. Alle, H. & Geiger, J.R. (2006) Combined analog and action potential coding in hippocampal mossy fibers. Science, 311, pp. 1290-1293. Allendoerfer, K.L. & Shatz, C.J. (1994) The subplate, a transient neocortical structure - its role in the development of connections between thalamus and cortex. Annual Review of Neuroscience, 17, pp. 185-218. Alvarez, P. & Squire, L.R. (1994) Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci U S A, 91, pp. 7041-7045. Amaral, D.G. (1979) Synaptic extensions from the mossy fibers of the fascia dentata. Anat Embryol (Berl), 155, pp. 241-251. Amaral, D.G., Ishizuka, N. & Claiborne, B. (1990) Neurons, Numbers and the Hippocampal Network. Understanding.the.Brain Through.the.Hippocampus., 83, pp. Amaral, D.G. & Witter, M.P. (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience, 31, pp. 571-591. Arabadzisz, D. & Freund, T.F. (1999) Changes in excitatory and inhibitory circuits of the rat hippocampus 12- 14 months after complete forebrain ischemia [In Process Citation]. Neuroscience, 92, pp. 27-45. Barnes, C.A., McNaughton, B.L., Mizumori, S.J., Leonard, B.W. & Lin, L.H. (1990) Comparison of spatial and temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Progress in brain research, 83, pp. 287-300. Baude, A., Nusser, Z., David, J., Roberts, B., Mulvihill, E., McIlhinney, R.A.J. & Somogyi, P. (1993) The metabotropic glutamate receptor (mGluR1Ó) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron, 11, pp. 771-787. Bayer, S.A. (1980) Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. The Journal of comparative neurology, 190, pp. 87-114. Bragin, A., Jando, G., Nadasdy, Z., van Landeghem, M. & Buzsaki, G. (1995) Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J Neurophysiol, 73, pp. 1691-1705. Brun, V.H., Otnass, M.K., Molden, S., Steffenach, H.A., Witter, M.P., Moser, M.B. & Moser, E.I. (2002) Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science, 296, pp. 2243-2246. Buckmaster, P.S. & Schwartzkroin, P.A. (1995) Interneurons and inhibition in the dentate gyrus of the rat in vivo. J Neurosci, 15, pp. 774-789. Butler, A.B. (1994) The evolution of the dorsal thalamus of jawed vertebrates, including mammals: cladistic analysis and a new hypothesis. Brain Res Brain Res Rev, 19, pp. 29-65. Buzsaki, G. (1989) Two-stage model of memory trace formation: A role for 'noisy' brain states. Neuroscience, 310, pp. 551-570. 40 Chawla, M.K., Guzowski, J.F., Ramirez-Amaya, V., Lipa, P., Hoffman, K.L., Marriott, L.K., Worley, P.F., McNaughton, B.L. & Barnes, C.A. (2005) Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus, 15, pp. 579-586. Chicurel, M.E. & Harris, K.M. (1992) 3-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. Journal of Comparative Neurology, 325, pp. 169-182. Chrobak, J.J. & Buzsaki, G. (1994) Selective activation of deep layer (V-VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J Neurosci, 14, pp. 6160-6170. Chrobak, J.J. & Buzsaki, G. (1996) High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci, 16, pp. 3056-3066. Claiborne, B.J., Amaral, D.G. & Cowan, W.M. (1986) A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J.Comp.Neurol., 246, pp. 435-458. Cobb, S.R., Halasy, K., Vida, I., Nyiri, G., Tamas, G., Buhl, E.H. & Somogyi, P. (1997) Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus [published erratum appears in Neuroscience 1997 Oct;80(3):971]. Neuroscience, 79, pp. 629648. Conrad, C.D. & Roy, E.J. (1993) Selective loss of hippocampal granule cells following adrenalectomy: implications for spatial memory. The Journal of neuroscience, 13, pp. 2582-2590. Costa, V.C., Bueno, J.L. & Xavier, G.F. (2005) Dentate gyrus-selective colchicine lesion and performance in temporal and spatial tasks. Behavioural brain research, 160, pp. 286-303. de la Iglesia, J.A., Martinez-Guijarro, F.I. & Lopez-Garcia, C. (1994) Neurons of the medial cortex outer plexiform layer of the lizard Podarcis hispanica: Golgi and immunocytochemical studies. The Journal of comparative neurology, 341, pp. 184-203. De Paola, V., Arber, S. & Caroni, P. (2003) AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nature neuroscience, 6, pp. 491-500. Debanne, D., Gahwiler, B.H. & Thompson, S.M. (1998) Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol, 507 ( Pt 1), pp. 237-247. Engel, D. & Jonas, P. (2005) Presynaptic action potential amplification by voltagegated Na+ channels in hippocampal mossy fiber boutons. Neuron, 45, pp. 405-417. Frank, L.M., Brown, E.N. & Wilson, M. (2000) Trajectory encoding in the hippocampus and entorhinal cortex. Neuron, 27, pp. 169-178. Fyhn, M., Molden, S., Witter, M.P., Moser, E.I. & Moser, M.B. (2004) Spatial representation in the entorhinal cortex. Science, 305, pp. 1258-1264. Geiger, J.R., Lubke, J., Roth, A., Frotscher, M. & Jonas, P. (1997) Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron, 18, pp. 1009-1023. Gilbert, P.E., Kesner, R.P. & Lee, I. (2001) Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus, 11, pp. 626-636. 41 Goffinet, A.M., Daumerie, C., Langerwerf, B. & Pieau, C. (1986) Neurogenesis in reptilian cortical structures: 3H-thymidine autoradiographic analysis. The Journal of Comparative Neurology, 243, pp. 106-116. Goodrich-Hunsaker, N.J., Hunsaker, M.R. & Kesner, R.P. (2005) Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behavioral neuroscience, 119, pp. 1307-1315. Gothard, K.M., Hoffman, K.L., Battaglia, F.P. & McNaughton, B.L. (2001) Dentate gyrus and ca1 ensemble activity during spatial reference frame shifts in the presence and absence of visual input. The Journal of neuroscience, 21, pp. 7284-7292. Gulyas, A.I., Megias, M., Emri, Z. & Freund, T.F. (1999) Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci, 19, pp. 10082-10097. Gulyas, A.I., Miettinen, R., Jacobowitz, D.M. & Freund, T.F. (1991) Calretininimmunoreacti cells in the rat hippocampus. I. A new type of neuron specifically associated with the mossy fibre system - revealed. Eur.J.Neurosci.Suppl., 4, pp. 158. Gulyas, A.I., Miles, R., Hájos, N. & Freund, T.F. (1993) Precision and variability in postsynaptic target selection of inhibitory cells in the hippocampal CA3 region. Eur J Neurosci, 5, pp. 1729-1751. Hafting, T., Fyhn, M., Molden, S., Moser, M.B. & Moser, E.I. (2005) Microstructure of a spatial map in the entorhinal cortex. Nature, 436, pp. 801-806. Hafting, T., Fyhn, M., Treves, A., Moser, E.I. & Moser, M.B. (2006) Coherent reallignment of entorhinal grid cells coincides global remapping in the hippocampus FENS, Vienna. Han, Z.S., Buhl, E.H., Lorinczi, Z. & Somogyi, P. (1993) A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. European Journal of Neuroscience, 5, pp. 395-410. Hargreaves, E.L., Rao, G., Lee, I. & Knierim, J.J. (2005) Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science, 308, pp. 1792-1794. Henze, D.A., Wittner, L. & Buzsaki, G. (2002) Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nature neuroscience, 5, pp. 790-795. Houser, C.R. & Esclapez, M. (1994) Localization of mRNAs encoding two forms of glutamic acid decarboxylase in the rat hippocampal formation. Hippocampus, 4, pp. 530-545. Isomura, Y., Sirota, A., Ozen, S., Montgomery, S., Mizuseki, K., Henze, D.A. & Buzsaki, G. (2006) Integration and segregation of activity in entorhinalhippocampal subregions by neocortical slow oscillations. Neuron, 52, pp. 871-882. Jeltsch, H., Bertrand, F., Lazarus, C. & Cassel, J.C. (2001) Cognitive performances and locomotor activity following dentate granule cell damage in rats: role of lesion extent and type of memory tested. Neurobiology of learning and memory, 76, pp. 81-105. Jonas, P., Major, G. & Sakmann, B. (1993) Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. Journal of Physiology - London, 472, pp. 615-663. Jung, M.W. & McNaughton, B.L. (1993) Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus, 3, pp. 165-182. 42 Kali, S. & Dayan, P. (2000) The involvement of recurrent connections in area CA3 in establishing the properties of place fields: a model. J Neurosci, 20, pp. 7463-7477. Kali, S. & Dayan, P. (2004) Off-line replay maintains declarative memories in a model of hippocampal-neocortical interactions. Nat Neurosci, 7, pp. 286-294. Kobayashi, K. & Poo, M.M. (2004) Spike train timing-dependent associative modification of hippocampal CA3 recurrent synapses by mossy fibers. Neuron, 41, pp. 445-454. Lassalle, J.M., Bataille, T. & Halley, H. (2000) Reversible inactivation of the hippocampal mossy fiber synapses in mice impairs spatial learning, but neither consolidation nor memory retrieval, in the Morris navigation task. Neurobiology of learning and memory, 73, pp. 243-257. Lawrence, J.J., Grinspan, Z.M. & McBain, C.J. (2004) Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. The Journal of physiology, 554, pp. 175-193. Lee, I. & Kesner, R.P. (2004) Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus, 14, pp. 66-76. Leutgeb, J.K., Leutgeb, S., Moser, M.B. & Moser, E.I. (2005a) Pattern separation in the dentate gyrus during morphing of two environments Society for Neuroscience, Washington, DC. Leutgeb, J.K., Leutgeb, S., Treves, A., Meyer, R., Barnes, C.A., McNaughton, B.L., Moser, M.B. & Moser, E.I. (2005b) Progressive transformation of hippocampal neuronal representations in "morphed" environments. Neuron, 48, pp. 345-358. Leutgeb, S., Leutgeb, J.K., Barnes, C.A., Moser, E.I., McNaughton, B.L. & Moser, M.B. (2005c) Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science, 309, pp. 619-623. Leutgeb, S., Leutgeb, J.K., Treves, A., Moser, M.B. & Moser, E.I. (2004) Distinct ensemble codes in hippocampal areas CA3 and CA1. Science, 305, pp. 12951298. Li, X.G., Somogyi, P., Ylinen, A. & Buzsaki, G. (1994) The hippocampal ca3 network - an in vivo intracellular labeling study. Journal of Comparative Neurology, 339, pp. 181-208. Lopez-Garcia, C. & Martinez-Guijarro, F.J. (1988) Neurons in the medial cortex give rise to Timm-positive boutons in the cerebral cortex of lizards. Brain research, 463, pp. 205-217. Maccaferri, G., Toth, K. & McBain, C.J. (1998) Target-specific expression of presynaptic mossy fiber plasticity. Science, 279, pp. 1368-1370. Marr, D. (1971) Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci, 262, pp. 23-81. Martinez Guijarro, F.J., Berbel, P.J., Molowny, A. & Lopez Garcia, C. (1984) Apical dendritic spines and axonic terminals in the bipyramidal neurons of the dorsomedial cortex of lizards (Lacerta). Anatomy and embryology, 170, pp. 321-326. Martinez-Guijarro, F.J., Soriano, E., Del Rio, J.A. & Lopez-Garcia, C. (1991) Zincpositive boutons in the cerebral cortex of lizards show glutamate immunoreactivity. Journal of neurocytology, 20, pp. 834-843. Maurer, A.P., Vanrhoads, S.R., Sutherland, G.R., Lipa, P. & McNaughton, B.L. (2005) Self-motion and the origin of differential spatial scaling along the septo-temporal axis of the hippocampus. Hippocampus, 15, pp. 841-852. 43 McClelland, J.L., McNaughton, B.L. & O'Reilly, R.C. (1995) Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev, 102, pp. 419-457. McNaughton, B.L., Barnes, C.A., Meltzer, J. & Sutherland, R.J. (1989) Hippocampal granule cells are necessary for normal spatial learning but not for spatiallyselective pyramidal cell discharge. Experimental brain research. Experimentelle Hirnforschung, 76, pp. 485-496. McNaughton, B.L. & Morris, N.G. (1987) Hippocampal synaptic enhancement and information storage within a distributed memory system. TINS, 10, pp. 408415. Megias, M., Emri, Z., Freund, T.F. & Gulyas, A.I. (2001) Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience, 102, pp. 527-540. Miles, R., Toth, K., Gulyas, A.I., Hájos, N. & Freund, T.F. (1996) Differences between somatic and dendritic inhibition in the hippocampus. Neuron, 16, pp. 815-823. Miles, R. & Wong, R.K. (1986) Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J.Physiol., 373, pp. 397-418. Molnar, Z. (2000) Development and evolution of thalamocortical interactions. Eur J Morphol, 38, pp. 313-320. Mori, M., Abegg, M.H., Gahwiler, B.H. & Gerber, U. (2004) A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature, 431, pp. 453-456. Moser, E.I., J.K., L., S., L. & M.-B., M. (2006) Pattern separation in the dentate gyrus and CA3 FENS, Vienna. Muller, R.U., Kubie, J.L. & Ranck, J.B., Jr. (1987) Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. The Journal of neuroscience, 7, pp. 1935-1950. Nadasdy, Z., Hirase, H., Czurko, A., Csicsvari, J. & Buzsaki, G. (1999) Replay and time compression of recurring spike sequences in the hippocampus [In Process Citation]. J Neurosci, 19, pp. 9497-9507. Nieuwenhuys, R. (1994) The neocortex. An overview of its evolutionary development, structural organization and synaptology. Anatomy and embryology, 190, pp. 307-337. Nitz, D. & McNaughton, B. (2004) Differential modulation of CA1 and dentate gyrus interneurons during exploration of novel environments. J Neurophysiol, 91, pp. 863-872. Northcutt, R.G. & Kaas, J.H. (1995) The emergence and evolution of mammalian neocortex. Trends in neurosciences, 18, pp. 373-379. O'Keefe, J. & Dostrovsky, J. (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res., 34, pp. 171175. O'Keefe, J. & Nadel, L. (1978) The hippocampus as a cognitive map. Clarendon, Oxford. O'Reilly, R.C. & McClelland, J.L. (1994) Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus, 4, pp. 661-682. Penttonen, M., Kamondi, A., Sik, A., Acsady, L. & Buzsaki, G. (1997) Feed-forward and feed-back activation of the dentate gyrus in vivo during dentate spikes and sharp wave bursts. Hippocampus, 7, pp. 437-450. 44 Quirk, G.J., Muller, R.U., Kubie, J.L. & Ranck, J.B., Jr. (1992) The positional firing properties of medial entorhinal neurons: description and comparison with hippocampal place cells. J Neurosci, 12, pp. 1945-1963. Rakic, P. (1995) A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends in neurosciences, 18, pp. 383388. Ramon y Cajal, S. (1911) Histologie de systeme nerveux de l’homme et des vertebres tomme II. Paris: Maloine. Reichova, I. & Sherman, S.M. (2004) Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol, 92, pp. 2185-2197. Rodriguez, F., Lopez, J.C., Vargas, J.P., Gomez, Y., Broglio, C. & Salas, C. (2002) Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. The Journal of neuroscience, 22, pp. 2894-2903. Rolls, E.T. (1989) Functions of neuronal networks in the hippocampus and cerebral cortex in memory. Models of brain function, pp. 15-33. Rolls, E.T. & Kesner, R.P. (2006) A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol, 79, pp. 1-48. Rumelhart, D.E. & Zipser, D. (1986) Feature discovery by competitive learning. In Rumelhart, D.E., McClelland, J., Group., P.R. (eds.) Parallel distributed processing: Explorations in the microstructure of cognition. Vol. 1. Foundations. MIT Press, Cambridge, MA, pp. 151-193. Salin, P.A., Scanziani, M., Malenka, R.C. & Nicoll, R.A. (1996) Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A, 93, pp. 13304-13309. Samsonovich, A. & McNaughton, B.L. (1997) Path integration and cognitive mapping in a continuous attractor neural network model. J Neurosci, 17, pp. 5900-5920. Scharfman, H.E., Kunkel, D.D. & Schwartzkroin, P.A. (1990) Synaptic Connections of Dentate Granule Cells and Hilar Neurons - Results of Paired Intracellular Recordings and Intracellular Horseradish Peroxidase Injections. Neuroscience, 37, pp. 693-707. Scoville, W.B. & Milner, B. (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry, 20, pp. 11-21. Seress, L. (1988) Interspecies comparison of the hippocampal formation shows increased emphasis on the regio superior in the Ammon's horn of the human brain. Journal fur Hirnforschung, 29, pp. 335-340. Sharp, P.E. (1991) Computer simulation of hippocampal place cells. Psychobiology, pp. Sherman, S.M. & Guillery, R.W. (1998) On the actions that one nerve cell can have on another: distinguishing "drivers" from "modulators". Proc Natl Acad Sci U S A, 95, pp. 7121-7126. Sik, A., Penttonen, M. & Buzsaki, G. (1997) Interneurons in the hippocampal dentate gyrus: an in vivo intracellular study. Eur J Neurosci, 9, pp. 573-588. Sik, A., Penttonen, M., Ylinen, A. & Buzsaki, G. (1995) Hippocampal CA1 interneurons: An in vivo intracellular labeling study. J.Neurosci., 15(10), pp. 6651-6665. Sik, A., Tamamaki, N. & Freund, T.F. (1993) Complete axon arborization of a single CA3 pyramidal cell in the rat hippocampus, and its relationship with postsynaptic parvalbumin-containing interneurons. European Journal of Neuroscience, 5, pp. 1719-1728. 45 Skaggs, W.E., McNaughton, B.L., Wilson, M.A. & Barnes, C.A. (1996) Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus, 6, pp. 149-172. Squire, L.R. (1992) Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev, 99, pp. 195-231. Super, H., Martinez, A., Del Rio, J.A. & Soriano, E. (1998) Involvement of distinct pioneer neurons in the formation of layer-specific connections in the hippocampus. The Journal of neuroscience, 18, pp. 4616-4626. Super, H. & Uylings, H.B. (2001) The early differentiation of the neocortex: a hypothesis on neocortical evolution. Cerebral cortex (New York, N.Y., 11, pp. 1101-1109. Sutherland, R.J., Whishaw, I.Q. & Kolb, B. (1983) A behavioural analysis of spatial localization following electrolytic, kainate- or colchicine-induced damage to the hippocampal formation in the rat. Behavioural brain research, 7, pp. 133153. Tabuchi, E., Mulder, A.B. & Wiener, S.I. (2003) Reward value invariant place responses and reward site associated activity in hippocampal neurons of behaving rats. Hippocampus, 13, pp. 117-132. Tamas, G., Somogyi, P. & Buhl, E.H. (1998) Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci, 18, pp. 4255-4270. Tashiro, A., Dunaevsky, A., Blazeski, R., Mason, C.A. & Yuste, R. (2003) Bidirectional regulation of hippocampal mossy fiber filopodial motility by kainate receptors: a two-step model of synaptogenesis. Neuron, 38, pp. 773784. Ten Donkelaar, H. (1998) Reptiles In: The central nervous system of vertebrates (Nieuwenhuys R, Ten Donkelaar HJ, Nicholson C, eds). Springer, Berlin. Toth, K., Suares, G., Lawrence, J.J., Philips-Tansey, E. & McBain, C.J. (2000) Differential mechanisms of transmission at three types of mossy fiber synapse. The Journal of neuroscience, 20, pp. 8279-8289. Treves, A. & Rolls, E.T. (1992) Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus, 2, pp. 189-199. Treves, A. & Rolls, E.T. (1994) Computational analysis of the role of the hippocampus in memory. Hippocampus, 4, pp. 374-391. Vida, I. & Frotscher, M. (2000) A hippocampal interneuron associated with the mossy fiber system. Proc Natl Acad Sci U S A, 97, pp. 1275-1280. Willshaw, D.J. & Buckingham, J.T. (1990) An Assessment of Marr Theory of the Hippocampus As a Temporary Memory Store. Phil.Trans.R.Soc.London., 329, pp. Wilson, M.A. & Mcnaughton, B.L. (1993) Dynamics of the hippocampal ensemble code for space. Science, 261, pp. 1055-1058. Wittner, L., Henze, D.A., Zaborszky, L. & Buzsaki, G. (2006) Hippocampal CA3 pyramidal cells selectively innervate aspiny interneurons. The European journal of neuroscience, 24, pp. 1286-1298. Xavier, G.F., Oliveira-Filho, F.J. & Santos, A.M. (1999) Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: difficulties in "place strategy" because of a lack of flexibility in the use of environmental cues? Hippocampus, 9, pp. 668-681. 46 Figure Legends Figure 1. Electron micrographs of different terminal types along the mossy fibers in the CA3 region (A-C, E) and of a CA3 pyramidal cell terminal (D) for comparison. All electron micrographs have the same magnification. A, B, A small en passant terminal establishes a single asymmetrical synapse on a dendritic shaft, showing the characteristic long perforated postsynaptic density of small terminal types on interneurons (arrows). C, A filopodial extension of a mossy fiber terminal forms a synapse (arrow) with an Substance P receptor-immunoreactive interneuron. D, A CA3 pyramidal cell establishes asymmetrical synapse on a simple spine of a CA1 pyramidal neuron. E, A large, double-headed mossy fiber terminal forms multiple contacts (arrows) with thorny excrescences of a CA3 pyramidal cell. All active zones converged on the same pyramidal cell. The individual release sites are short. Scale bars: A-D, 0.5 µm; E, 1 µm. Reprinted with permission from Acsády et al., 1998, Society for Neuroscience. Figure 2. Filopodial extensions of mossy fiber terminals are specialized to innervate GABAergic cells. Artistic rendition of two large mossy terminals, each equipped with four filopodial extensions (large arrowheads). The mossy fibers were labeled by intracellular injection of biocytin into two neighboring granule cells. All filopodial terminals were examined in the electron microscope (not shown) and all contacted the dendrites or spines of altogether six GABAergic neurons. Four of the GABAergic neurons were identified by their Substance P receptor-content and two of them by ultrastructural characteristics. Five of the six postsynaptic interneurons were spiny cells. Arrows point to the main axons. Reprinted with permission from Acsády et al., 1998, Society for Neuroscience. 47 Figure 3. Spike transmission dynamics between a granule cell and its interneuron and pyramidal cell targets in CA3c in vivo. A) Camera lucida reconstruction of the extracellular electrode track and biocytin-labeled granule cell. Inset, a higher-power view of the mossy fiber axon near the probe track. Arrowheads, mossy fiber boutons. B) Superimposed (n = 60) intracellularly evoked action potentials in a granule cell (bottom traces) and simultaneously recorded extracellular units (filtered 0.8−8 kHz). Note the time-locked response of a putative pyramidal cell to the granule cell action potentials. C-D) Cross-correlograms between the evoked granule cell action potentials and the activity of a putative CA3c pyramidal cell (C) or interneuron (D). The values are shuffle corrected and expressed as probability (number of unit spikes per bin/total number of granule cell spikes). Arrowhead, peak time of the granule cell action potential. E) Representative results of the effect of intratrain frequency on spike transmission probability for a putative pyramidal cell. F) Spike transmission probability (shuffle-corrected probability of spike in 6 ms following granule cell spike) as a function of spike number in evoked 100 Hz train ( s.e.m). Solid line, putative interneurons (n = 24); dotted line, putative pyramidal cells (n = 21). Scale bars, A) 50 m; inset 20 m; B) 1 ms, 25 mV, 75 V. m, molecular layer; g, granule cell layer; h, hilus; IC, intracellular electrode track; EC, extracellular electrode track.