NOTES_CEGE1009_2
advertisement

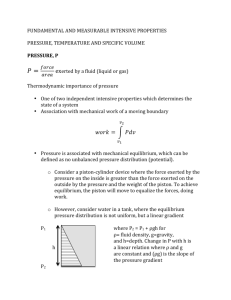
Lecture 2: Tuesday 11th January 2011 Review of Archimedes, Pascal Law, and derivation of hydrostatics equations 2.3 Application to fluids of constant density pressure-measuring devices: barometers, manometers, etc. 2.4 Application to fluids of variable density Tutorial Sheet No.2 9 CIVIL, ENVIRONMENTAL and GEOMATIC ENGINEERING DEPARTMENT 1st YEAR FLUIDS 2.3 CEGE1009 MECHANISMS Applications for fluids of constant density Ignoring any minor variations in g, if the fluid density is constant (as is generally the case for liquids), then the equation above can be integrated to give the widely used formula: p + g z = constant where the constant depends on the datum adopted for pressure measurements. For instance, if a liquid has its surface open to atmospheric pressure patm and z = 0 at that surface, then the pressure pz at any point distance z relative to datum is: Patm pz + g z = patm z +ve z=0 and at a depth h below the liquid surface: ph = patm + g h h +ve Pressure increases linearly with depth - and is independent of container shape. The "gauge pressure" is then given as: pgauge = ph - patm = g h h = pgauge / g is defined as the pressure head. Pressure differences in a liquid can thus be visualised in terms of length of a column of liquid. The term: p / g + z is called the piezometric head and p + g z is called the piezometric pressure. A piezometer tube, used to measure piezometric head in groundwater flow and other engineering applications, is a small tube open to the atmosphere at its top end - and of sufficient length to accommodate a free surface for the pressure head being measured. It is basically a method of determining the gauge pressure. However, it is not widely used because it cannot measure negative pressures, high gauge pressures (without an extremely long tube), or pressures in gases. h = P/g P+ z datum 10 2.3.1 Barometers If the top of a piezometer tube is closed off and the gas above the liquid surface evacuated to create a Pv perfect vacuum, then the height of liquid within the tube is now related to the absolute pressure - rather than to the gauge pressure (which, remember, is the difference between the pressure being measured and local atmospheric pressure). Barometers are based x on this principle, using mercury as the liquid because its density is sufficiently high for atmospheric pressure to be measured using a relatively short length of tube and because it has a C C’ very low vapour pressure at room temperature. The tube, filled with pure mercury, is inverted with its Mercury hg base immersed in a bath of pure mercury. The pressure at the horizontal surface through the free surface of the mercury in the bath C-C' is then Patm (in the bath) and PV + hg g x (in the tube), where PV is the vapour pressure (normally taken as negligible) at the top of the tube. Hence: Patm = PV + hg g x ≈ hg g x x is normally about 750 mm. However, an equivalent water barometer would need a tube over 10 m long. 2.3.2 Manometers Manometers record the pressure difference between two points using columns of a (relatively dense) liquid. The difference may be between one point in the fluid and atmospheric pressure, or between two points within the fluid. They do not suffer from the constraints applying to the simple piezometer tube - negative pressures and high gauge pressures are no problem, and they can measure pressures in gases. Its simplest form is the U-tube manometer. This consists of a glass (or clear plastic) U-tube set in a vertical plane against a graduated scale rule and containing a liquid (B) immiscible with and of greater density than the fluid whose pressure is being measured. Within a continuous expanse of the same stationary fluid the pressure is the same at any two points in a horizontal plane. Hence, in the figure shown, the pressures in the two arms at level C-C' must be the same: Patm Fluid A (light) x P+ y C C’ p + A g y = patm + B g x where B is the density of the manometer liquid and A is the density of the fluid being measured. Fluid B (heavier) Hence, pgauge = p - patm = B g x - A g y If the fluid A is a gas, then B >> A and pgauge can be approximated by: pgauge = B g x 11 Such devices are also frequently used to measure pressure differences across flow restrictions like orifice plates and venturimeters. Again, pressure in arm C = pressure in arm C'. Orifice plate Fluid A P1 flow P2 y Hence, p1+ A g(x + y) = p2 + A g y + B g x and p1 - p2 = ( B - A ) g x which is independent of y - that is, the location of the manometer tube relative to the position of the measurements. If A is a liquid, then it is sometimes convenient to express the result in terms of head: x C’ C Liquid B ( p1 p2 ) = ( B 1 ) x = h1 h2 A g A A very common error is to forget the ... - 1 in this equation. Remember that it actually represents the pressure exerted by the column of fluid A. On the other hand, if A is a gas, with B >> A p1 - p2 = B g x The Reservoir manometer is a modification of the system described above, intended for simplified use. It has one arm enlarged to such an extent that the movement of the meniscus in it is negligible compared to that in the narrow arm. This might be achieved by increasing the cross-sectional area by a factor of 400 (that is, the diameter is 20 times larger), thus effectively turning the enlarged arm into a reservoir. With such an instrument, it is necessary only to measure the change of level in the narrow arm. P 2 Scale rule P 1 x If it is necessary to make accurate measurements, the movement in the manometer arm should be as large as possible. One way to increase the sensitivity is for the narrow arm of a reservoir manometer to be set on a shallow slope. In this way, a small change in pressure is reflected as a relatively large movement in the meniscus along the arm. Such a device is known as an inclined reservoir manometer. 12 P2 s P1 x C’ C Here, the actual movement of manometer liquid up the arm, s, is related to the vertical displacement, x, by: x = s sin Hence: p1 - p2 = B g x = B g s sin which shows that, for a given pressure difference, s must increase for smaller angles of inclination . Another method of measuring small differences in pressure is to use an inverted U-tube manometer, with a liquid slightly less dense than liquid A. Again considering pressures at level C-C': p1 - A g y = p2 - A g (y - x - (z2 - z1 )) - B g x Only if B A Fluid B C’ C x (p1 + A g z1 ) - (p2 + A g z2 ) = g x (A - B ) or: p 1 - p 2 = g x ( A - B ) * where p* are piezometric pressures. For a given pressure difference, x will get larger if the difference in densities (A - B ) is kept very small. 2.3.3 y * P1 Liquid A z1 P2 Orifice plate z2 Other pressure gauges There are a number of other (perhaps more convenient) techniques for measuring pressure. For instance, electronic pressure transducers include a small flexible diaphragm (a few millimetres in diameter) which is exposed to the fluid pressure on one side. Vibrating crystals, wires, or strain gauges detect any deflections of the diaphragm from the "dry" side, and signal processors calibrate the output in terms of pressure. Such devices can be designed to measure very high or small pressures, and to respond rapidly or slowly - depending on requirements. The Bourdon Gauge consists of a curved, hollow elastic tube of elliptical cross-section, clamped at one end but allowed to move at the other. When the tube is pressurised, it tries to take up a circular cross-section and therefore tends to change in curvature. By connecting a dial gauge indicator needle to one end of the tube, the needle moves round the dial as the tube changes shape under the action of the applied pressure. 13 2.4 Applications for fluids of variable density ( - gases with density varying with pressure) Starting from hydrostatics: the fundamental equation z isopleths of P0-5p p + g = 0 z P0-4p If the atmosphere (or other gas) is considered "perfect", then: p = RT Earth’s surface, where p=P0 p pg + z RT P0-3p P0-2p P0-p P0 = 0 Integration to give a relationship between pressure and height requires additional information or an assumption about the variation of T with z. a) For an isothermal atmosphere, assume T constant = T 0 say, and assume g constant also. dp g + dz = 0 p R T0 integrating: log e p + gz = constant k = log e p0 RT0 with p = p0 at z = 0. Then: log e p gz =; RT0 p0 g z p = e ( RT0 ) p0 b) For an atmosphere with a temperature lapse rate Temperature decreases with altitude and, by definition, T = T 0 - z (in practice, has been found to be 0.0065 K/m for about 9 km above the earth's surface) Then, as above, p g dz p g dz + = + =0 p RT p R ( T 0 z) z Stratosphere 32k m Troposphere integrating, with p = p0 at z = 0 9km log e p g g log e ( T 0 z) = log e p0 log e ( T 0 ) R R 14 T 2230 K log e p g T z ) = log e ( 0 p0 R T0 and p z g = (1 )R p0 T0 For small values of z / T0 this equation may be expanded by the Binomial Rule to show: gz p g = 1 z + ...... = 1 0 R T0 p0 p0 Hence: p = p 0 - 0 g z A similar finding can also be deduced by expanding the equivalent equation in the case of an isothermal atmosphere. What this demonstrates is that for small heights (z < 300 m, say ), the atmosphere behaves like a fluid of constant density with a constant variation of pressure with z. However, in dealing with gases on the length scale of a few metres, the gravitational term contributes negligibly and pressure over a vertical surface can normally be taken as constant. This can be demonstrated by considering the pressure change over a 10 m high wall: 0 g z = 1.23 x 9.81 x 10 = 121 Pa whereas atmospheric pressure is 1.01 x 10 5 Pa 15 CEGE1009 Mechanisms (Fluids): TUTORIAL SHEET 2 Density of water w = 1000 kg / m3 ; of mercury hg = 13560 kg/m3 ; R (air) = 287 J / ( kg K ) 1. A tank 3.5 m long and 2.5 m wide contains alcohol of relative density 0.82 to a uniform depth of 3 m. A 50 mm diameter pipe leads from the bottom of the tank. Determine the readings (in Pa) on a gauge connected at the following points: a) 150 mm above the bottom of the tank b) in the 50 mm diameter pipe, 2 m below the bottom of the tank c) at the upper end of a 25 mm diameter pipe, connected to the 50 mm pipe 2 m below the bottom of the tank, and sloping upwards at 300 to the horizontal for 1.2 m and then rising vertically for 600 mm. Also calculate the load on the bottom of the tank. [Answer: 22.93 kPa, 40.2 kPa, 30.57 kPa, 211.2 kN] 2. An inverted U-tube manometer is used to measure the pressure drop across a sudden contraction in a horizontal stretch of water supply pipeline. If the actual pressure loss is found to be 294 Pa, calculate the reading on the inverted manometer if the fluid above the water is i) air, and ii) oil with density 800 kg/m3. [Answer: 30 mm, 150 mm ] 3. The figure below shows a special manometer arranged so that a difference in pressure between A and B will move the surface of separation in C. The cross-sectional areas of reservoirs A, B and the Utube are given as 500 mm2, 800 mm2, and 70 mm2, and the densities of the liquids in A and B are 800 kg/m3 and 900 kg/m3 respectively. Find the pressure difference which will produce a rise in C of 60 mm. 4. A rigid vessel of capacity 0.1 m3 contains air at a gauge pressure of 345 kPa and a temperature of 300 C. Atmospheric pressure is 752 mm of mercury. Calculate the mass of air in the vessel. If the atmospheric pressure rises to 765 mm mercury and the temperature falls to -100 C, calculate the new gauge pressure. [Answer: 0.512 kg, 284.5 kPa ] 5. Assuming that atmospheric temperature decreases with increasing altitude at a uniform rate of 0.0065 K/m, determine the atmospheric pressure at an altitude of 7500 m if the temperature and pressure at sea level are 150 C and 101.5 kPa respectively. [Answer: 38.3 kPa ] 16